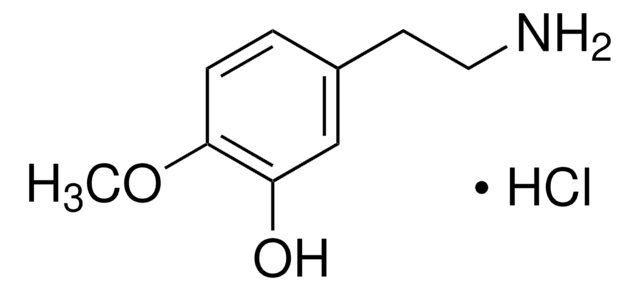
187305
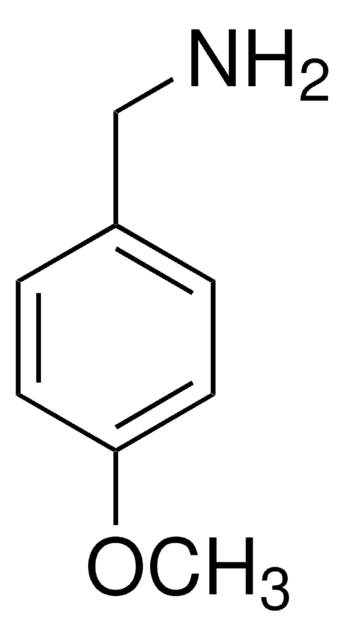
4-Methoxyphenethylamine
≥98%
Synonym(s):
2-(4-Methoxyphenyl)ethylamine, 4-Methoxyphenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
151.21
Beilstein:
508967
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.538 (lit.)
bp
138-140 °C/20 mmHg (lit.)
254-256 °C
density
1.031 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
COc1ccc(CCN)cc1
InChI
1S/C9H13NO/c1-11-9-4-2-8(3-5-9)6-7-10/h2-5H,6-7,10H2,1H3
InChI key
LTPVSOCPYWDIFU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Methoxyphenethylamine inhibits the monoamine oxidase-catalyzed deamination of both tyramine and tryptamine.
4-Methoxyphenethylamine is used as a precursor for the synthesis of other organic compounds by the alkylation reaction.
4-Methoxyphenethylamine is used as a precursor for the synthesis of other organic compounds by the alkylation reaction.
Application
4-Methoxyphenethylamine was used in the synthesis of :
- pyrrolo[3,2-c]carbazole
- poly(4-methoxyphenethylamine), required for the immobilization of nitrogenated bases and oligonucleotides
- organopolyphosphazenes such as poly[bis(4-methoxy benzylamino)polyphosphazene] and poly[bis(4-methoxyphenethylamino)polyphosphazene]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characterization of novel polyorganophosphazenes substituted with 4-methoxybenzylamine and 4-methoxyphenethylamine for in vitro release of indomethacin and 5-fluorouracil.
Gudasi KB, et al.
Reactive functional Polymers, 66(10), 1149-1157 (2006)
W J Keller et al.
Journal of pharmaceutical sciences, 65(10), 1539-1540 (1976-10-01)
It has been established that the oxidative deamination of tyramine by monoamine xodase is inhibited by (+/-)-4-methoxy-beta-hydroxyphenethylamine and its N-methylated derivatives. This particular series of compounds does not inhibit the action of monoamine oxidase when tryptamine is used as the
Electrochemical Investigation of oligonucleotide-DNA hybridization on poly(4-methoxyphenethylamine).
Francielle B Silva et al.
International journal of molecular sciences, 9(7), 1173-1188 (2009-03-28)
This work describes the immobilization of purine and pyrimidine bases and immobilization/hybridization of synthetic oligonucleotides on graphite electrodes modified with poly(4-methoxyphenethylamine) produced in acid medium. The immobilization of adenine, guanine, cytosine and thymine on these modified electrodes was efficient, producing
The Pummerer cyclization route to the ibophyllidine alkaloids. Total synthesis of (?)-deethylibophyllidine.
Catena J, et al.
Tetrahedron Letters, 35(25), 4433-4436 (1994)
T Kuramoto et al.
Journal of neuroscience research, 6(1), 37-48 (1981-01-01)
K+-sensitive liquid ion exchange electrode systems respond with a slow potential change to acetylcholine, choline, anticholinergic drugs, biogenic amines, and glutamic acid. The response threshold has been defined, and in most cases it is at extremely low concentrations (10(-7)-10(-5)M). The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service