187062
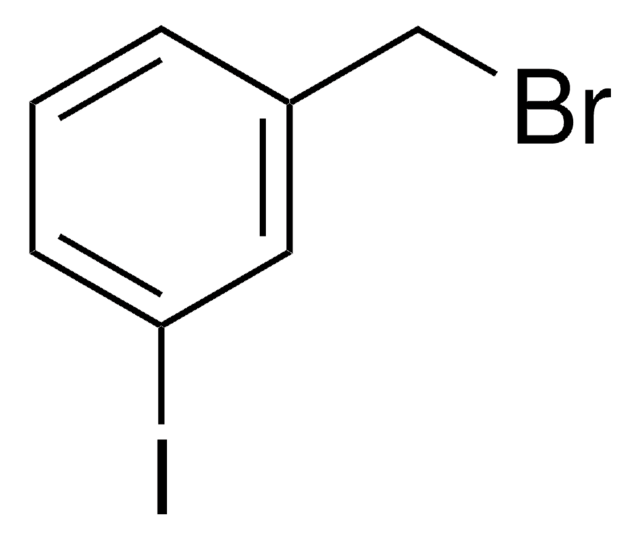
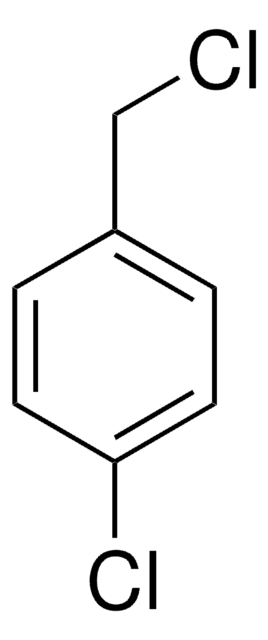
3-Bromobenzyl bromide
99%
Synonym(s):
α,3-Dibromotoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4CH2Br
CAS Number:
Molecular Weight:
249.93
Beilstein:
2078683
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
39-41 °C (lit.)
functional group
bromo
SMILES string
BrCc1cccc(Br)c1
InChI
1S/C7H6Br2/c8-5-6-2-1-3-7(9)4-6/h1-4H,5H2
InChI key
ZPCJPJQUVRIILS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Bromobenzyl bromide undergoes reduction with diethylzinc in the presence of Pd(PPh3)4 to yield corresponding hydrocarbon.
Application
3-Bromobenzyl bromide was used in the synthesis of:
- 1,7-di(3-bromobenzyl)cyclen
- substituted 8-arylquinoline, phosphodiesterase 4 (PDE4) inhibitors
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of a new family of bi-and polycyclic compounds via Pd-catalyzed amination of 1, 7-di (3-bromobenzyl) cyclen.
Averin AD, et al.
Tetrahedron Letters, 49(24), 3950-3954 (2008)
Reduction of benzylic halides with diethylzinc using tetrakis (triphenylphosphine) palladium as catalyst.
Agrios KA and Srebnik M.
The Journal of Organic Chemistry, 58(24), 6908-6910 (1993)
Dwight Macdonald et al.
Bioorganic & medicinal chemistry letters, 15(23), 5241-5246 (2005-09-20)
The discovery and SAR of a new series of substituted 8-arylquinoline PDE4 inhibitors are herein described. This work has led to the identification of several compounds with excellent in vitro and in vivo profiles, including a good therapeutic window of
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 34-43 (2008-12-25)
For the successful implementation of Distributed Drug Discovery (D(3)) (outlined in the accompanying Perspective), students, in the course of their educational laboratories, must be able to reproducibly make new, high quality, molecules with potential for biological activity. This article reports
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service