158194
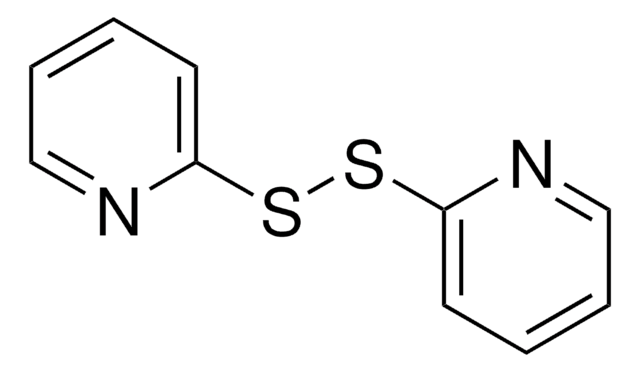
2,2′-Dithiobis(5-nitropyridine)
96%
Synonym(s):
Bis(5-nitro-2-pyridyl) disulfide, DTNP
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6N4O4S2
CAS Number:
Molecular Weight:
310.31
Beilstein:
305413
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
155-157 °C (lit.)
functional group
disulfide
nitro
SMILES string
[O-][N+](=O)c1ccc(SSc2ccc(cn2)[N+]([O-])=O)nc1
InChI
1S/C10H6N4O4S2/c15-13(16)7-1-3-9(11-5-7)19-20-10-4-2-8(6-12-10)14(17)18/h1-6H
InChI key
ROUFCTKIILEETD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,2′-Dithiobis(5-nitropyridine) is an aromatic disulphide.
Application
2,2′-Dithiobis(5-nitropyridine) was employed:
- as cysteine-activating reagent to study the NMR of G protein-coupled receptors
- in the deprotection assays for protected selenocysteine-containing peptides
- for deprotecting p-methoxybenzyl groups and acetamidomethyl groups from the side-chains of cysteine and selenocysteine
- to remove the p-methoxybenzyl protecting group from cysteine and selenocysteine side-chains
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G J Stephens et al.
Pflugers Archiv : European journal of physiology, 431(3), 435-442 (1996-01-01)
The effects of cysteine-modifying reagents on the gating of rat cloned Kv1.4 channels expressed in HEK-293 cells were examined using the whole-cell patch-clamp technique. Cells transfected with Kv1.4 expressed a rapidly inactivating K+ current with a mid-point of activation of
A Li et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 18(17), 6740-6747 (1998-08-26)
Functional modifications of neuronal P/Q-type voltage-dependent Ca2+ channels expressed in Xenopus oocytes by oxidation were examined electrophysiologically. Oxidation by external H2O2 enhanced the whole-oocyte currents through the Ca2+ channels composed of the alpha1A, alpha2/delta, and beta3 subunits at negative voltages
Leah S Cohen et al.
Biopolymers, 102(1), 16-29 (2013-07-31)
Structural analysis by NMR of G protein-coupled receptors (GPCRs) has proven to be extremely challenging. To reduce the number of peaks in the NMR spectra by segmentally labeling a GPCR, we have developed a Guided Reconstitution method that includes the
P Sharma et al.
Analytical biochemistry, 189(2), 173-177 (1990-09-01)
A sensitive and simple method is described for the quantitative determination of free sulfhydryl (-SH) groups on polymer supports. The method includes the reaction of 4,4'-dimethoxytrityloxy-S-(2-thio-5-nitropyridyl)-2-mercapto ethane (DTNPME) with polymer-supported sulfhydryl groups. After removal of excess reagent through washing, a
Alayne L Schroll et al.
Journal of peptide science : an official publication of the European Peptide Society, 18(1), 1-9 (2011-11-16)
Of all the commercially available amino acid derivatives for solid phase peptide synthesis, none has a greater abundance of side-chain protection diversity than cysteine. The high reactivity of the cysteine thiol necessitates its attenuation during peptide construction. Moreover, the propensity
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 158194-10G | 4061832544670 |
| 158194-1G | 4061825970103 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service