Duolink® Proximity Ligation Assay (PLA) Multicolor Kits for Sensitive Multiplex Detection
Duolink® PLA Kits and Reagent Packs for Multicolor Visualization and Quantification of Protein-protein Interactions
Protein-protein Interaction Events
Interacting proteins often function as key components in the initiation and cascade of inter-and intracellular communications and influence a large variety of cellular functions and behaviors. However, accurately identifying protein-protein interactions in fixed tissues and cells can be challenging, especially for low abundant proteins or weekly interacting protein complexes. Due to the discovery of a growing list of fluorophores and novel detection technologies, diverse fields of research have noted tremendous advancements due to the identification of intracellular protein interactions. As a result, the detection of these interactions has led to improvements in our understanding of protein function and have provided key insight into numerous signaling pathways in healthy and diseased tissues. Duolink® PLA Multicolor technology allows for the visual detection and quantification of several distinct protein-protein interactions simultaneously in fixed tissues and cells.1
Duolink® PLA Multicolor Technology
Duolink® PLA Multicolor Probemaker kits and reagent packs deliver an effective solution for visually identifying and quantifying up to four distinct protein-protein interactions within a variety of fixed cells and tissues. Two highly specific primary antibodies are required for the evaluation of each protein-protein interaction. The generation of a plus and minus PLA probe is accomplished using Probemaker technology and involves directly conjugating a single oligo to each primary antibody. If each plus and minus PLA probe pair are near each other (<40nM), DNA ligation followed by rolling circle amplification occurs with the addition of polymerase and produces a connected single-stranded concatemeric sequence with up to 1000 copies of the template. The addition of labeled oligos or detection probes hybridize to the complementary template sequences within the amplicon resulting in up to a 1000x protein signal amplification for each protein detection event. Multiplex Duolink® PLA Multicolor technology allows for the detection of both stable and weakly interacting proteins for up to four protein events in detection colors consisting of green, orange, red, and far red (Figure 1). Importantly, both imaging and data analysis are achievable with the use of standard high-content screening imagers, flow cytometry, or imaging flow cytometers.

Figure 1:Directly conjugated primary antibodies (PLA probes), generated with Probemaker technology, are used to identify and amplify signals for four unique protein events in green, orange, red, and far red detection colors.
Duolink® PLA Multicolor Technology and Cellular Pathway Analyses
Investigating protein-protein interactions and cellular pathway analyses includes a variety of methods and technologies. Commonly used fluorophores and fluorescent microscopy may involve the use of recombinant proteins and the colocalization of signals within the cell or tissue of interest. However, the use of recombinant proteins and colocalization imaging techniques are limited and may not provide data for interacting proteins in their native state. Furthermore, studying various diseases where complex signaling pathways are involved may include the analysis of proteins with subtle post-translational modifications or the detection of phosphorylated and non-phosphorylated proteins. Duolink® PLA Multicolor technology is effective at analyzing protein-protein interactions with potential post-translational or alternative modifications.
Investigation of the HER Family of Receptor Tyrosine Kinases Using Multiplex Duolink® PLA Multicolor Technology
The HER family of receptor tyrosine kinases consists of four members: EGFR (HER1 or ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). Importantly, these proteins are overexpressed or mutated in numerous types of cancer and are of great interests to researchers.2 Provided the high specificity and the ability to investigate up to four protein-protein interactions simultaneously, Multiplex Duolink® PLA Multicolor technology can be effectively used to visualize complex signaling cascades, including the EGFR pathway (Figure 2).
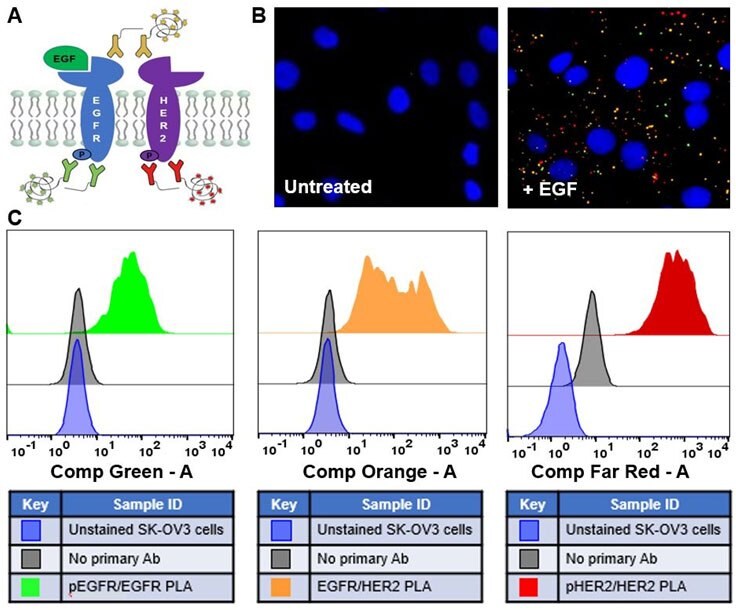
Figure 2:(A) Schematic of multiplex PLA detection and analysis of EGFR signaling of post-translational modifications and protein-protein interactions in SK-OV3 cells treated with EGF (+ EGF) or untreated cells. Multiplex PLA detection of the phosphorylation of EGFR (green), EGFR-HER2 interactions (orange), and the phosphorylation of HER2 (far red) were performed on adherent cells (B) or detached cells (C). Data collection was performed by fluorescence microscopy or flow cytometry, respectively. Nuclei were stained with DAPI (blue) in (B).
Refer to the Duolink® PLA Multicolor Detection protocol for further detailed information on executing an experiment.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?