Ellman's Sulfinamides
Since its introduction by Ellman in 1997 as a chiral ammonia equivalent,1 enantiopure 2-methyl-2-propanesulfinamide (tert-butanesulfinamide) has been demonstrated to be a versatile chiral auxiliary and has found extensive use both in academics and industry. Condensation of tert-butanesulfinamide with aldehydes and ketones proceeds under mild conditions and provides tert-butanesulfinyl imines in high yields. The tert-butanesulfinyl group activates these imines for the addition of many different classes of nucleophiles and serves as a powerful chiral directing group to provide products with generally high diastereoselectivity. Subsequent removal of the tert-butanesulfinyl group under mild conditions cleanly provides the amine products.
These tert-butanesulfinyl imines have been used as intermediates in the asymmetric synthesis of many versatile building blocks2 including syn- and anti- 1,2- or 1,3-amino alcohols,3,4 α-branched and α,α-dibranched amines,5 and α- or β-amino acids and esters6,7 (Scheme 1). Several researchers have taken advantage of the robust chemistry of tert-butanesulfinyl imines in the synthesis of antibiotics, biologically active compounds and other complex natural products.8 Furthermore, tert-butanesulfinyl imines have been used in the synthesis of asymmetric ligands,9 and in a few cases, appears as the chirality-bearing component.10

Scheme 1.Asymmetric synthesis of many versatile building blocks using tert-butanesulfinyl imines
Recently, tert-butanesulfinyl imines have been employed in the synthesis of chiral heterocycles. A few groups have synthesized chiral aziridines through a common tert-butanesulfinyl imine intermediate (Scheme 2). Morton and co-workers synthesized chiral aziridines using trimethylsulfonium iodide with good yields and diastereoselectivities.11a Chemla and Ferreira reacted a racemic allenylzinc substrate with various tert-butanesulfinyl imines to achieve trans-ethynylaziridines as diastereomerically and enantiomerically pure compounds in good yields.11b

Scheme 2.synthesis of chiral aziridines through a common tert-butanesulfinyl imine intermediate
Additionally, Dondas and De Kimpe devised an efficient route to pyrrolidines and piperidines using a common racemic tert-butanesulfinyl amine (Scheme 3), which is easily achieved from the sulfinyl imine by reduction with NaBH4.12 Their synthesis highlights a one-pot cascade cyclization and fragmentation, which allows for very high yields and purity of the cyclized product.

Scheme 3.Pyrrolidines and piperidines using a common racemic tert-butanesulfinyl amine
Ellman’s research group has also reported the synthesis of chiral heterocycles. In an extension of their work on synthesis of 1,3-amino-alcohols,4a Ellman carried out the asymmetric syntheses of (-)-halosaline and (-)-8-epihalosaline (Scheme 4).

Scheme 4.Asymmetric syntheses of (-)-halosaline and (-)-8-epihalosaline
Another recent report describes the intermolecular self-condensation of chiral tert-butanesulfinyl imines in a synthesis for the pyrrolizidine alkaloid SC-53116 (Scheme 5).13 In a later report, Ellman demonstrated the facile synthesis of chiral 2-substituted pyrrolidines (Scheme 6) that proceeds with high yields and diastereoselectivities.14

Scheme 5.Self-condensation of chiral tert-butanesulfinyl imines to form pyrrolizidine alkaloid
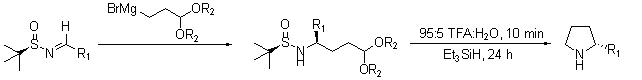
Scheme 6.Facile synthesis of chiral 2-substituted pyrrolidines
We are pleased to be able to offer this versatile and useful auxiliary in both enantiomeric and racemic forms for your research.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?