Good Cell Banking Practices
ECACC Laboratory Handbook 4th Edition

It is poor practice to maintain a cell line in continuous or extended culture for the following reasons:
- Risk of microbial contamination
- Loss of characteristics of interest (e.g. surface antigen or monoclonal antibody expression)
- Genetic drift particularly in cells known to have an unstable
karyotype (e.g. CHO, BHK 21) - Loss of cell line due to exceeding finite lifespan (e.g. human diploid cells such as MRC-5)
- Risk of cross contamination with other cell lines
- Increased consumables and staff costs
All of these potential risk factors may be minimized by the implementation of a cell banking system (Figure 1). This type of system is known as a tiered banking system or master cell banking system. On initial arrival into the laboratory, a new cell culture should be regarded as a potential source of contamination (e.g. harboring bacteria, fungi and mycoplasma and should be handled under quarantine conditions until proven negative for such microbial contaminants). Following initial expansion, 3-5 ampoules should be frozen as a token stock before a master cell bank is prepared. One of the token stock ampoules should then be thawed and expanded to produce a master cell bank of 10-20 ampoules depending upon the anticipated level of use.
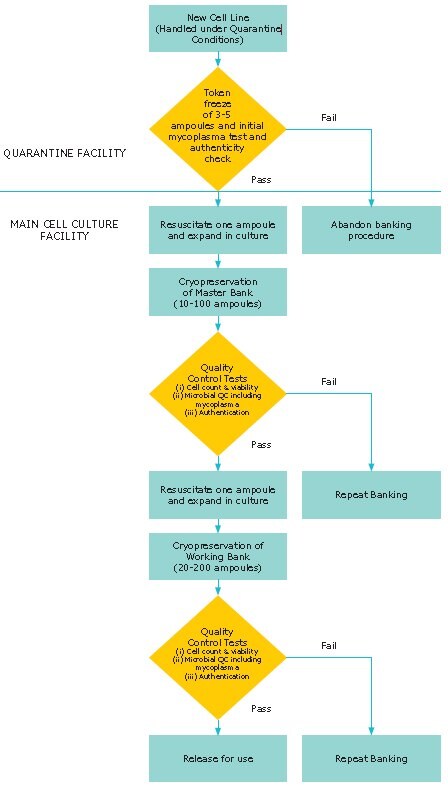
Figure 1.Schematic Representation of a Tiered Cell Banking System
Ampoules of this master cell bank (2-3) should be allocated for quality control confirmation that the cell count and viability of the bank is acceptable and that the bank is free of bacteria, fungi, and mycoplasma. It is also important at this stage to authenticate the master cell bank by STR profiling, DNA barcoding, or SNP analysis. Additional tests, such as viral screening, are also required. Once these tests have been completed satisfactorily an ampoule from the master cell bank should be thawed and cultured to produce a working cell bank. The size of this bank will again depend on the envisaged level of demand. Quality control tests (cell count, viability, authentication, and the absence of microbial contaminants) are again required prior to using the cultures for routine experimentation or production.
Important note: Authenticate the Master and Working Cell Banks by STR profiling, DNA barcoding, or SNP analysis.
Implementation of this banking system ensures:
- Material is of a consistent quality
- Experiments are performed using cultures in the same range of passage numbers
- Cells are only in culture when required
- The original cell line characteristics are retained
Notes
- The number of ampoules prepared for master and working cell banks depends upon the forecast demand for their use.
- The number of ampoules sampled for quality control is dependent upon the size of bank. Ideally, 5% of the bank should be tested before use.
- Ampoules from the working cell bank should be used sequentially, keeping cells in culture for not more than a predetermined number of cell doublings. This number will be least in the case of cell lines having a finite life-span (e.g. diploid lines).
- The working cell bank should be replenished from an ampoule of the master cell bank. This should be done in sufficient time to allow the quality control to be completed.
- A new master cell bank should be prepared before the number of original master stock drops below five ampoules.
- The panel of quality control tests performed depends upon the use intended e.g. regulatory authorities may require additional tests such as viral screening and karyotypic studies.
如要继续阅读,请登录或创建帐户。
暂无帐户?