Improving HILIC Mode Chromatography by Choosing the Proper Injection and Wash Solvent
Introduction to HILIC
HILIC chromatography, also known as Aqueous Normal Phase (ANP), uses a polar stationary phase such as bare silica, cyano, amino, phenyl, pentafluorophenyl (PFPP) or diol and a relatively non-polar mobile phase. The mobile phase usually consists of a high percentage of organic with water and buffer as the modifier. Typical analytes run in this mode are basic amines (polar and non-polar), polar acids and polar neutrals. HILIC is a form of partitioning, the water in the mobile phase is preferentially adsorbed to the polar stationary phase resulting in a layer of solvent enriched in water near the surface and a layer of solvent enriched in organic in the bulk mobile phase. The analyte is distributed between these two layers. A more polar solute will partition into the water layer and thus be retained longer than a less polar solute. HILIC also can include hydrogen bonding and ion-exchange directly with the surface.
HILIC has been gaining popularity in recent years due to the increased retention of polar compounds and the alternative selectivity HILIC chromatography offers to reversed-phase. In addition, the HILIC mode is highly compatible with mass spectrometric (MS) and evaporative light scattering (ELSD) detection due to the volatility of the mobile phase. Further, HILIC is advantageous to use in preparative chromatography due to the relative ease of compound recovery from a volatile mobile phase. Higher flow rates are also possible due to the lower viscosity of the mobile phase. In terms of sample prep, high organic solid phase extraction (SPE) eluents can be directly injected into the HPLC without further evaporation and re-constitution. Finally, HILIC is useful in metabolite separations. As compounds are metabolized, they become more polar and retention by reversed-phase is difficult. HILIC will retain these polar compounds longer, allowing for easier separation and identification of these metabolites.
As HILIC chromatography may be thought of as reversed reversed-phase, there are a number of operational details that the analyst must be aware of when preceding with this form of chromatography. This work describes how the autosampler needle wash solvent can adversely affect the chromatography.
Discussion
Figure 1 shows a typical QA chromatogram that would be obtained with an Ascentis Express HILIC HPLC column. Figure 2 is a chromatogram obtained from a new, unused Ascentis Express HILIC column on a Waters® Acquity® UPLC system. Note the difference from the results obtained in Figure 1. Several attempts were made to troubleshoot the problem, such as washing with strong solvent (water), examining the end frit, running on another system, reversing flow direction, altering sample injection solvents, and running a larger diameter column (4.6 mm I.D.). None of these changes solved the problem.

Conditions for Figures 1-3 (53930-U)
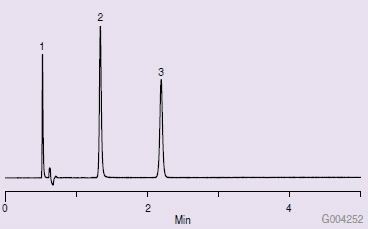
Figure 1.Typical QA Chromatogram for Ascentis Express HILIC Column
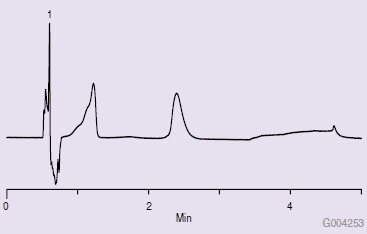
Figure 2.Chromatogram from First Injection on a New Column (wrong technique)
In troubleshooting the poor chromatography, a review of the manufacturer’s literature showed the instrument had several injection modes. The partial loop mode was used to generate the chromatogram in Figure 2, as this was the default method set in the instrument. In this partial loop mode, it was reported in the manufacturer’s literature that this is the only mode that injects sample and weak wash solvent onto the column.1 Normally, this would be an acceptable mode to inject samples if the mode was reversed-phase LC. In HILIC mode, however, solvent and sample polarities are opposite versus reversed- phase mode.
This Acquity UPLC instrument has two wash solvents, strong and weak. When the instrument was initially installed, the strong wash solvent was 10:90 water:acetonitrile and the weak wash solvent was 90:10 water:acetonitrile. The instrument was injecting a small volume of the weak wash solvent (90:10 water:acetonitrile) along with the sample. This weak wash solvent is actually a strong wash solvent in HILIC mode.
To determine if this wash solvent was causing the poor chromatography, the needle wash solvent bottles were reversed. Upon changing solvent strengths in the needle wash and making another injection, the chromatogram in Figure 3 was obtained, indicating the needle wash solvent polarity was the cause of the poor chromatography.
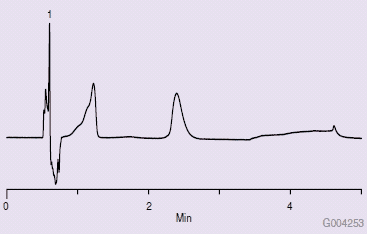
Figure 3.Chromatogram After Switching Wash Solvent Bottles Between Weak and Strong Solvents
To determine if this solved the poor chromatography problem, the needle wash solvent bottles were switched back to their initial configuration. The resulting chromatogram was similar to that shown in Figure 2, indicating the poor chromatography could be reproduced. In addition to the strong and weak wash solvent polarities, other injection modes in the UPLC were investigated. Results from changing injection modes showed chromatography similar to that seen in Figure 3, even using the original wash solvent system.
Solvent needle wash polarity has been shown to play a vital role in HILIC chromatography. In addition, injection mode also played a role in obtaining good chromatography. In the work presented here, the column was initially suspected to be bad. Further investigation showed the needle wash solvents to be critical in the HILIC mode as well as the sample injection mode in obtaining good chromatography in this alternative chromatographic mode.
When using HILIC chromatography, especially after running an LC in reversed-phase mode, it is important to remember to check the LC to be certain needle wash solvents, sample injection type and sample solvents are compatible with HILIC mode so that errors in column viability are not made.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?