S7576
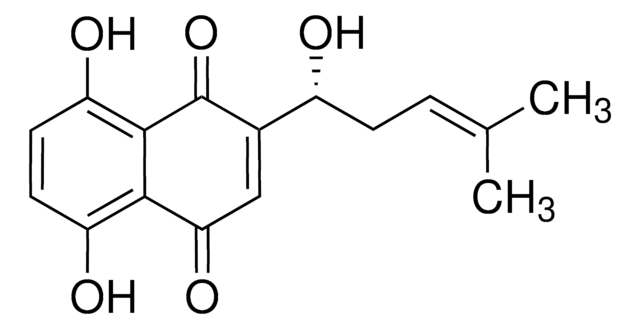
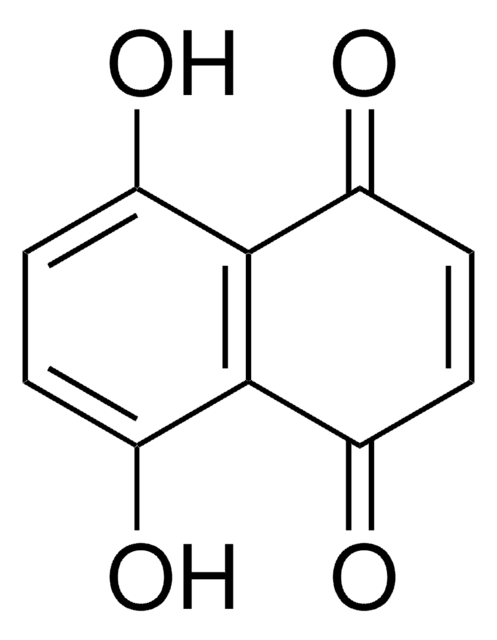
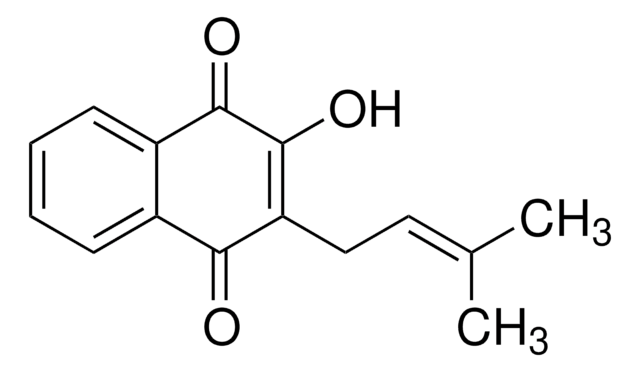
Shikonin
≥98% (HPLC)
Synonym(s):
(±)-5,8-Dihydroxy-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4-naphthoquinone, (±)-Alkannin, (±)-Shikalkin, (±)-Shikonin
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder or solid
color
red to brown
solubility
methanol: 1 mg/mL
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
C\C(C)=C\CC(O)C1=CC(=O)c2c(O)ccc(O)c2C1=O
InChI
1S/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3
InChI key
NEZONWMXZKDMKF-UHFFFAOYSA-N
Related Categories
General description
Application
- to enhance the anti-tumor activity of gefitinib on tyrosine kinase receptor epidermal growth factor receptor (EGFR) in wild-type lung cancer cells
- as an inhibitor of renal aerobic glycosis and a potential
- as an anti-inflammatory agent to test its role to improve lipopolysaccharide (LPS)-induced cardiac dysfunction
- to stimulate bone marrow-derived macrophages (BMDMs) to test the effect of Interferon regulatory factor 1 on cell death
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article about how proliferating cells require the biosynthesis of structural components for biomass production and for genomic replication.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service