P1431
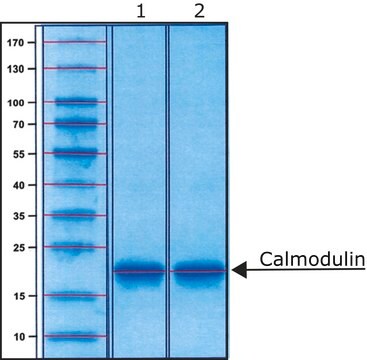
Calmodulin from bovine testes
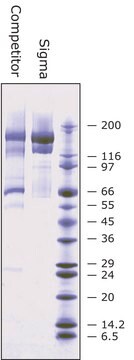
BioUltra, ≥98% (SDS-PAGE), lyophilized powder, essentially salt free
Synonym(s):
CaM, Phosphodiesterase 3′:5′-cyclic nucleotide activator
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
biological source
bovine testis
Quality Level
product line
BioUltra
Assay
≥98% (SDS-PAGE)
form
lyophilized powder
mol wt
16.79 kDa
storage condition
(Keep container tightly closed in a dry and well-ventilated place)
technique(s)
ligand binding assay: suitable
impurities
salt, essentially free
UniProt accession no.
application(s)
cell analysis
storage temp.
−20°C
Gene Information
cow ... CALM3(520277)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Research area: Cell Signaling
Calmodulin (CaM) is a Ca2+-sensor protein containing four EF-hand motifs that bind to four Ca2+ ions. It is found ubiquitously in all eukaryotes.
Calmodulin (CaM) is a Ca2+-sensor protein containing four EF-hand motifs that bind to four Ca2+ ions. It is found ubiquitously in all eukaryotes.
Application
Calmodulin from bovine testes has been used:
- as a component of the reaction mixture in PhosphoSens assay to measure Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) substrate phosphorylation
- to generate standard curve for the determination of in situ calmodulin concentration in tissues
- as a ligand in radio-ligand binding for studying calmodulin affinity
Biochem/physiol Actions
Calmodulin (CaM) aids in the Ca2+ signal transduction pathway in higher plants and animals. Ca2+ binding is required for CaM activation. Upon activation, CaM binds and activates numerous target proteins involved in a variety of cellular processes including regulation of plant metabolism, phytohormone signaling, ion transport, protein folding, protein phosphorylation and dephosphorylation, cell motility, exocytosis, and cytoskeletal assembly. In neurons, calcium-activated CaM helps in the regulation of glutamate receptors, modulation of proteins in signaling pathways, and regulation of voltage-gated calcium channels (VGCCs) activity.
Ca2+ binding protein that is required for activation of cyclic nucleotide-dependent phosphodiesterase. It is also a cofactor/activator of nitric oxide synthase, calcineurin, and many kinases including ATPase, myosin light chain kinase, and CAM kinase I, II, and III. It mediates ryanodine receptor activation by cyclic ADP ribose and is involved in intracellular Ca2+ homeostasis.
antibody
Product No.
Description
Pricing
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The diversity of calcium sensor proteins in the regulation of neuronal function
McCue HV, et al.
Cold Spring Harbor Perspectives in Biology, 2(8) (2010)
comparative proteomics illustrates the molecular mechanism of potato (Solanum tuberosum L.) tuberization inhibited by exogenous gibberellins in vitro
Cheng L, et al.
Physiologia Plantarum, 163, 103-123 (2018)
Arkadiusz Miazek et al.
Scientific reports, 11(1), 7312-7312 (2021-04-02)
The neuronal membrane-associated periodic spectrin skeleton (MPS) contributes to neuronal development, remodeling, and organization. Post-translational modifications impinge on spectrin, the major component of the MPS, but their role remains poorly understood. One modification targeting spectrin is cleavage by calpains, a
E J McConnell et al.
Circulation research, 86(2), 191-197 (2000-02-10)
Plasma membrane (Ca(2+)+Mg(2+))-ATPase and Ca(2+) transport activities, best characterized in human erythrocytes, are stimulated by calmodulin and thought to play a crucial role in the termination of cellular Ca(2+) signaling in all cells. In plasma membranes isolated from cultured porcine
Elisha R Injeti et al.
American journal of physiology. Heart and circulatory physiology, 295(6), H2289-H2298 (2008-10-07)
Postnatal decreases in vascular reactivity involve decreases in the thick filament component of myofilament calcium sensitivity, which is measured as the relationship between cytosolic calcium concentration and myosin light chain (MLC20) phosphorylation. The present study tests the hypothesis that downregulation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service