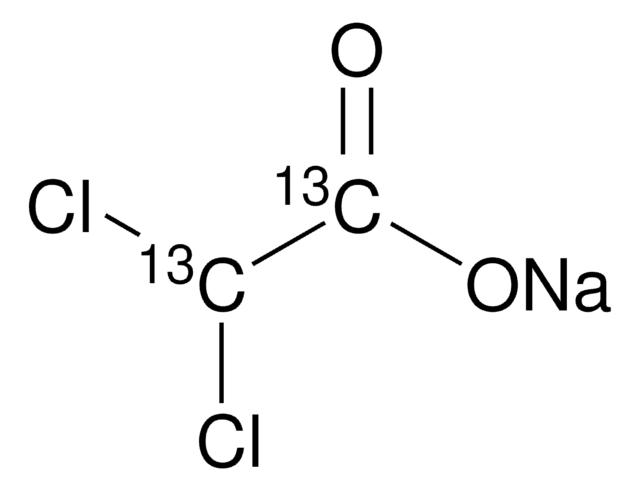
O2751
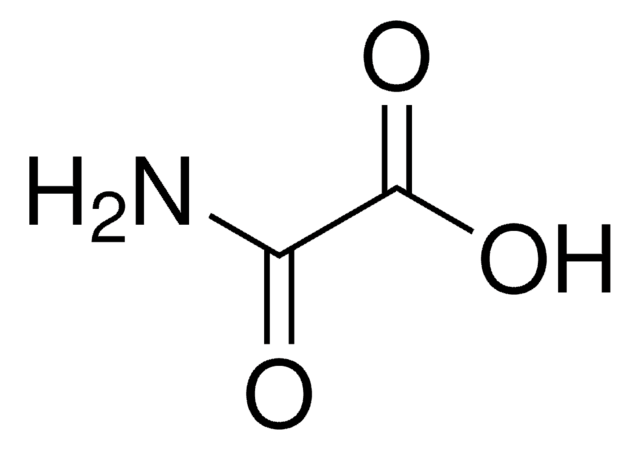
Sodium oxamate
≥98%
Synonym(s):
Aminooxoacetic acid sodium salt, Oxalic acid monoamide sodium salt, Oxamic acid sodium salt
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98%
form
powder
mp
300 °C
solubility
water: 50 mg/mL, clear, colorless
SMILES string
[Na+].NC(=O)C([O-])=O
InChI
1S/C2H3NO3.Na/c3-1(4)2(5)6;/h(H2,3,4)(H,5,6);/q;+1/p-1
InChI key
RQVZIJIQDCGIKI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- as one of the variable in flow injection analysis
- in in vitro angiogenesis assay
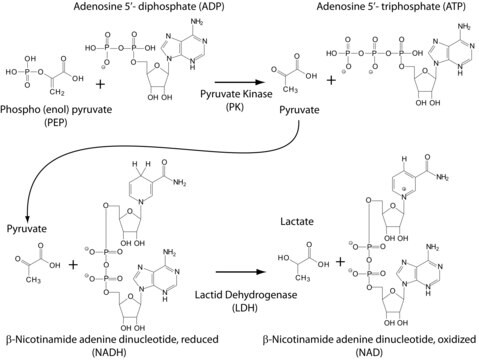
- to confirm the stimulatory role of stearic acid on lactate dehydrogenase mediated lactate production
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service