MP0035
LookOut® Mycoplasma PCR Detection Kit
Optimized for use with JumpStart™ Taq DNA Polymerase, D9307.
Synonym(s):
PCR-based mycoplasma screening
About This Item
Recommended Products
quality
Not suitable for clinical diagnostic use. Will not detect clinically relevant species such as M. pneumoniae and U. urealyticum
Quality Level
packaging
pkg of 1 kit
technique(s)
PCR: suitable
application(s)
detection
microbiology
compatibility
Optimized for use with JumpStart™ Taq DNA Polymerase, D9307.
storage temp.
2-8°C
General description
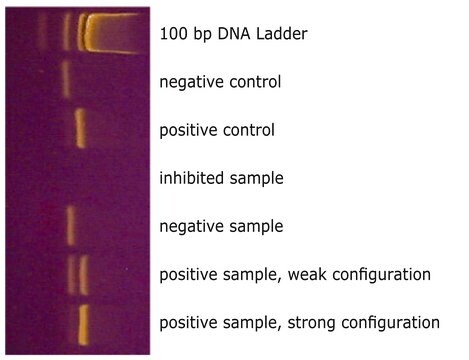
The reaction tubes included with the kit are pre-coated with appropriate dNTPs, primers, and loading dye. Total assay time is greatly reduced compared to general protocols that require individual loading of reaction tubes.
Application
Compatibility
Legal Information
related product
Storage Class Code
10 - Combustible liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Detect mycoplasma contamination in cell cultures three ways: culture test, DNA stain test, or mycoplasma PCR test. Mycoplasma elimination kits rid cultures of contamination.
Protocols
Mycoplasma contamination of cell cultures is a serious issue impacting cell model validity. PCR testing for mycoplasma is an inexpensive, sensitive, and specific method for detecting contamination.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service