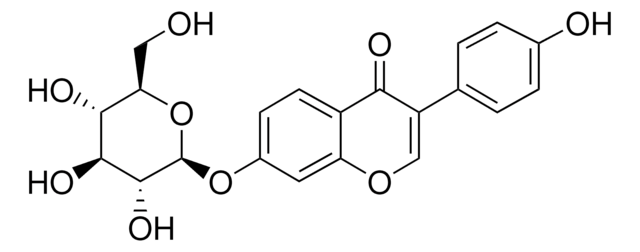
G1296
Glycitin
≥98% (HPLC)
Synonym(s):
4′,7-Dihydroxy 6-methoxyisoflavone 7-O-glucoside, 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-3-(4-hydroxyphenyl)-6-methoxy-, Glycitein 7-O-β-glucoside
About This Item
Recommended Products
biological source
plant (Pueraria thunbergianaI)
Assay
≥98% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
white to faint beige
mp
203 - 204 °C ((397 - 399 °F ))
solubility
10 mg, clear, colorless to faintly yellow
storage temp.
room temp
SMILES string
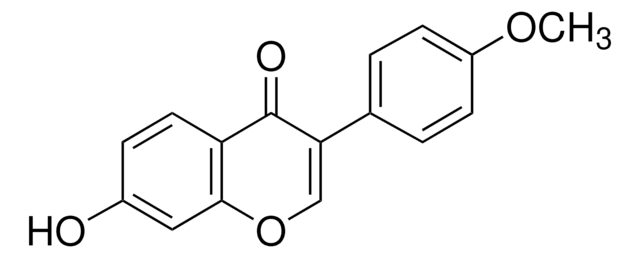
O=C1C2=CC(OC)=C(O[C@H]3[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O3)C=C2OC=C1C4=CC=C(O)C=C4
InChI
1S/C22H22O10/c1-29-15-6-12-14(30-9-13(18(12)25)10-2-4-11(24)5-3-10)7-16(15)31-22-21(28)20(27)19(26)17(8-23)32-22/h2-7,9,17,19-24,26-28H,8H2,1H3/t17-,19-,20+,21-,22-/m1/s1
InChI key
OZBAVEKZGSOMOJ-MIUGBVLSSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells.: This study highlights Glycitin′s therapeutic potential in mitigating intervertebral disc degeneration by counteracting inflammatory and oxidative processes, demonstrating its viability as a bioactive compound for degenerative diseases (Zhao W et al., 2023).
- Spectrum-effect relationship study to reveal the pharmacodynamic substances in Flos Puerariae-Semen Hoveniae medicine pair for the treatment of alcohol-induced liver damage.: Analyzes the pharmacological effects of herbal components, including Glycitin, targeting liver damage recovery, illustrating the compound′s therapeutic relevance in traditional and modern medical applications (Zhang H et al., 2023).
Biochem/physiol Actions
Packaging
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service