C6423
α-Chymotrypsin from bovine pancreas
suitable for protein sequencing, salt-free, lyophilized powder
About This Item
Recommended Products
grade
Proteomics Grade
Quality Level
form
salt-free, lyophilized powder
mol wt
25 kDa
suitability
suitable for protein sequencing
UniProt accession no.
storage temp.
2-8°C
Gene Information
cow ... CTRB1(618826)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Protein Identification by mass spectrometry (MS)
- The isolation and characterization of myosin heavy chains
- Toxin preparation
- The incubation of infected and uninfected cells for analysis of cellular proteins by SDS-PAGE
Biochem/physiol Actions
Unit Definition
Other Notes
inhibitor
substrate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
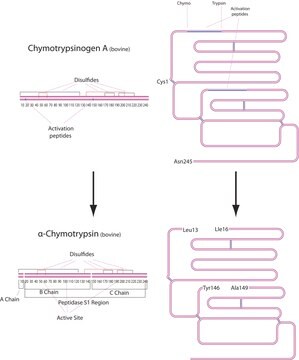
Analytical Enzyme Chymotrypsin: Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service