C1999
CMP-Sialic Acid Synthetase from Neisseria meningitidis group B
recombinant, expressed in E. coli BL21, ≥10 units/mg protein
Synonym(s):
CTP: N-Acylneuraminate cytidylyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized solid
specific activity
≥10 units/mg protein
mol wt
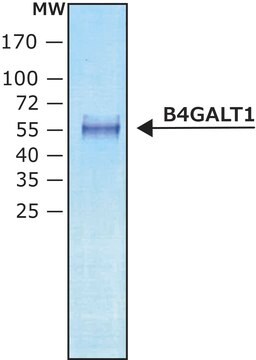
26.0 kDa
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Cytidine monophosphate (CMP)-Sialic Acid Synthetase from Neisseria meningitidis group B is encoded in neuA gene. The protein has a molecular weight of 24.8 kDa.
Application
The enzyme has been utilized to synthesize CMP-sialic acid and its derivatives.
Biochem/physiol Actions
Cytidine monophosphate (CMP)-sialic acid synthetase catalyses the conversion of N?acetylneuraminic acid (NeuNAc) to CMP-NeuNAc. CMP-sialic acid synthetase has globular α/β domain and is categorised under αβα three-layered sandwich fold. The dimerization domain aids the interaction between the monomers. It also has mononucleotide binding and NeuAc binding pocket. Mg2+ is essential for the catalytic functionality of CMP-sialic acid synthetase.
Unit Definition
One unit will catalyze the formation of 1 μmol CMP-Neu-5-Ac from Neu-5-Ac and CTP per minute at 37 °C at pH 8.0.
Physical form
Supplied as a lyophilized powder containing Tris-HCl and NaCl.
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 8.5) containing Neu-5-Ac (1 mM) and CTP (1 mM) at 37 °C for 30 min and analyzed using capillary electrophoresis with a UV detector (200 nm).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Molecular cloning and analysis of genes for sialic acid synthesis in Neisseria meningitidis group B and purification of the meningococcal CMP-NeuNAc synthetase enzyme.
Ganguli S, et al.
Journal of Bacteriology, 176(15), 4583-4589 (1994)
Thomas Haselhorst et al.
Biochemical and biophysical research communications, 359(4), 866-870 (2007-06-19)
We report an easy and direct application of 'Saturation Transfer Double Difference' (STDD) NMR spectroscopy to identify ligands that bind to a Sepharose-immobilised target protein. The model protein, cytidine 5'-monophosphate sialic acid (CMP-Sia) synthetase, was expressed as a Strep-Tag II
Structure of a sialic acid-activating synthetase, CMP-acylneuraminate synthetase in the presence and absence of CDP
Mosimann S, et al.
The Journal of biological chemistry, 276(11), 8190-8196 (2001)
Rahman M Mizanur et al.
Applied microbiology and biotechnology, 76(4), 827-834 (2007-07-03)
In this study, we report the cloning, recombinant expression, and biochemical characterization of a heat-stable CMP-N-acylneuraminic acid (NeuAc) synthetase from Clostridium thermocellum ATCC 27405. A high throughput electrospray ionization mass spectrometry (ESI-MS)-based assay demonstrates that the enzyme has an absolute
Rahman M Mizanur et al.
Applied microbiology and biotechnology, 80(5), 757-765 (2008-08-22)
Sialic acids are abundant nine-carbon sugars expressed terminally on glycoconjugates of eukaryotic cells and are crucial for a variety of cell biological functions such as cell-cell adhesion, intracellular signaling, and in regulation of glycoproteins stability. In bacteria, N-acetylneuraminic acid (Neu5Ac)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service