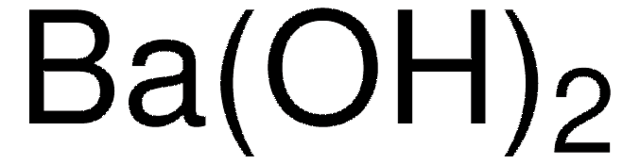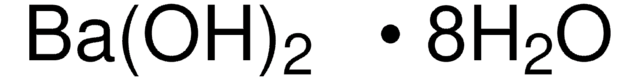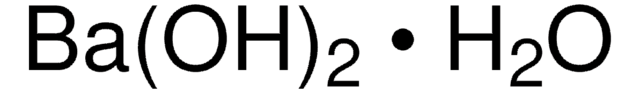All Photos(3)
About This Item
Linear Formula:
Ba(OH)2 · H2O
CAS Number:
Molecular Weight:
189.36
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
Assay:
98%
form:
powder, crystals or chunks
Recommended Products
vapor pressure
300 hPa ( 78 °C)
Quality Level
Assay
98%
form
powder, crystals or chunks
density
3.743 g/mL at 25 °C (lit.)
SMILES string
O.O[Ba]O
InChI
1S/Ba.3H2O/h;3*1H2/q+2;;;/p-2
InChI key
GKQTUHKAQKWLIN-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M F Byford
The Biochemical journal, 280 ( Pt 1), 261-265 (1991-11-15)
The beta-elimination of phosphoserine residues by dilute alkali is catalysed by the presence of group II metal ions. The use of 0.1 M-Ba (OH)2 catalysed the rate of beta-elimination of phosphoserine by more than two orders of magnitude compared with
Spontaneous ignition, explosion, and fire with sevoflurane and barium hydroxide lime.
Junzheng Wu et al.
Anesthesiology, 101(2), 534-537 (2004-07-28)
J Tanaka et al.
Dental materials journal, 12(1), 1-11 (1993-06-01)
This study investigated the durability, especially the nonwaterdegradable qualities, of experimental light-cured composite resin containing barium-borosilicate glass filler. For this purpose, Bis-GMA, a typical component of base monomer in conventional composite resin, was replaced by Bis-GMA-F which is water-repellent. After
P J Baxter et al.
Anesthesiology, 89(4), 929-941 (1998-10-20)
Desflurane, enflurane and isoflurane can be degraded to carbon monoxide (CO) by carbon dioxide absorbents, whereas sevoflurane and halothane form negligible amounts of CO. Carbon monoxide formation is greater with drier absorbent, and with barium hydroxide, than with soda lime.
T Irie et al.
Carbohydrate research, 192, 167-172 (1989-10-23)
The alkylation of cyclomalto-oligosaccharides (cyclodextrins, CDs) with dialkyl sulfate-barium hydroxide has been claimed to yield 2,6-di-O-alkyl derivatives. Re-investigation by plasma desorption-m.s. of the products of laboratory methylation of alpha CD, beta CD, or gamma CD and ethylation of beta CD
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service