70372
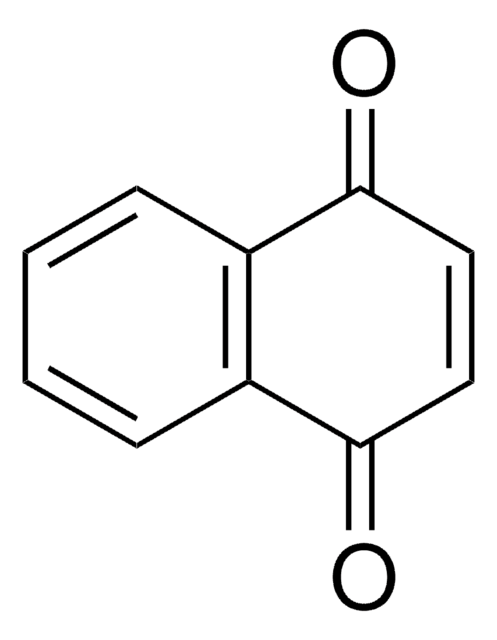
1,4-Naphthoquinone
purum, ≥96.5% (HPLC)
Synonym(s):
α-Naphthoquinone
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥96.5% (HPLC)
form
powder
mp
119-122 °C (lit.)
120-124 °C
functional group
ketone
SMILES string
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
InChI key
FRASJONUBLZVQX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
It can be used to synthesize:
- 3,3-Disubstituted oxindoles via asymmetric Michael addition to oxindole.
- Bioactive isoindolines via asymmetric 1,3-dipolar cycloaddition to azomethine ylides generated in situ from aldehydes and diethyl aminomalonate.
- α,α-Difluoro-β-hydroxy ketone via ‘on water′ catalyst-free Mukaiyama-aldol reaction with difluoroenoxysilane.
- 2-Hydroxy-3-anilino-1,4-naphthoquinone, which shows potent in vivo antimalarial activity.
Additional appilcation include:
- As an arylation reagent for the α-arylation of aldehydes.
- As a starting material in the multi-step synthesis of benz[f]indole-4,9-diones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
285.8 °F
Flash Point(C)
141 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Chlorobenzilate; 4-Aminobiphenyl; 2-Fluorobiphenyl; N-Nitrosopyrrolidine; 1,2,4,5-Tetrachlorobenzene; 3-Methylcholanthrene; Phenacetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service