03440
N-Ethyldiisopropylamine
≥98.0%
Synonym(s):
N,N-Diisopropylethylamine, Hünigs base
About This Item
Recommended Products
Quality Level
Assay
≥98.0%
form
liquid
impurities
≤0.5% water
refractive index
n20/D 1.414
bp
126-128 °C (lit.)
density
0.755 g/mL at 20 °C (lit.)
0.755 g/mL at 20 °C
0.757 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCN(C(C)C)C(C)C
InChI
1S/C8H19N/c1-6-9(7(2)3)8(4)5/h7-8H,6H2,1-5H3
InChI key
JGFZNNIVVJXRND-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Catalytic activity in dioxygen reduction: A study utilized Mn complexes with pendent proton donor relays and added base, including N-Ethyldiisopropylamine, to control product selectivity during dioxygen reduction, providing insights into catalytic mechanisms and potential industrial applications (Cook et al., 2024).
- Three-dimensional imaging in medical applications: N-Ethyldiisopropylamine was mentioned in the context of its role in improving the resolution of three-dimensional imaging techniques based on computed tomography angiography (CTA), crucial for preoperative perforator selection in reconstructive surgery (Su et al., 2024).
- Synthesis of Spirocyclopropane-Containing Compounds: Research demonstrated the application of N-Ethyldiisopropylamine in the synthesis of spirocyclopropane-containing 4H-pyrazolo[1,5-a]indoles through alkylative dearomatization and intramolecular N-imination, highlighting its utility in complex organic synthesis (Huang et al., 2023).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.1 °F
Flash Point(C)
9.5 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service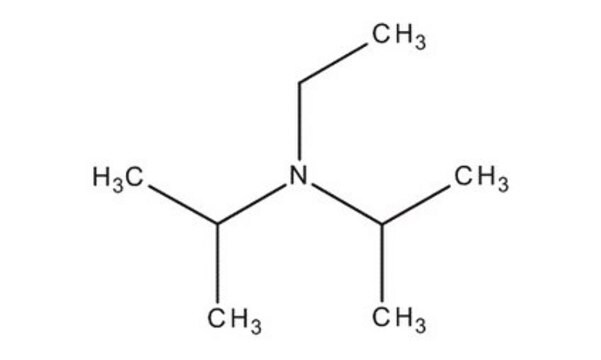

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)