T72761
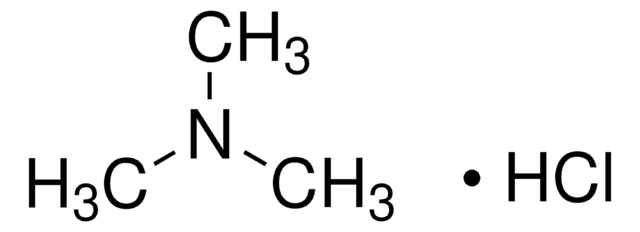
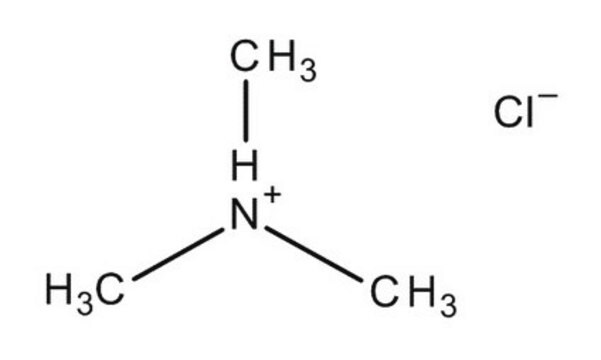
Trimethylamine hydrochloride
98%
Synonym(s):
Trimethylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
(CH3)3N · HCl
CAS Number:
Molecular Weight:
95.57
Beilstein:
3905063
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
283-284 °C (dec.) (lit.)
SMILES string
Cl[H].CN(C)C
InChI
1S/C3H9N.ClH/c1-4(2)3;/h1-3H3;1H
InChI key
SZYJELPVAFJOGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Trimethylamine hydrochloride is a precursor to prepare aluminium chloride- trimethylamine hydrochloride ionic liquid, which is commonly used in electrodeposition of aluminum wires. It is also used in the synthesis of trimethylamine gallane, trimethylamine-borane and azido-bridged perovskite-type metal-organic frameworks (MOFs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
GaN@ ZIF-8: Selective formation of gallium nitride quantum dots inside a zinc methylimidazolate framework.
Esken D, et al.
Journal of the American Chemical Society, 133(41), 16370-16373 (2011)
Danny Orabi et al.
JCI insight, 6(9) (2021-05-15)
Gut microbe-derived metabolites influence human physiology and disease. However, establishing mechanistic links between gut microbial metabolites and disease pathogenesis in animal models remains challenging. The major route of absorption for microbe-derived small molecules is venous drainage via the portal vein
Electrodeposition of aluminum wires from the Lewis acidic AlCl3/trimethylamine hydrochloride ionic liquid without using a template.
Su C-J, et al.
Electrochemical Communications, 34, 170-173 (2013)
Electrodeposition of purified aluminum coatings from dimethylsulfone-AlCl3 electrolytes with trimethylamine hydrochloride.
Miyake M, et al.
Surface and Coatings Technology, 206(19-20), 4225-4229 (2012)
Structures and Aggregation of the Methylamine?Borane Molecules, MenH3?nN?BH3 (n = 1?3), Studied by X-ray Diffraction, Gas-Phase Electron Diffraction, and Quantum Chemical Calculations.
Aldridge S, et al.
Journal of the American Chemical Society, 131(6), 2231-2243 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service