E45708
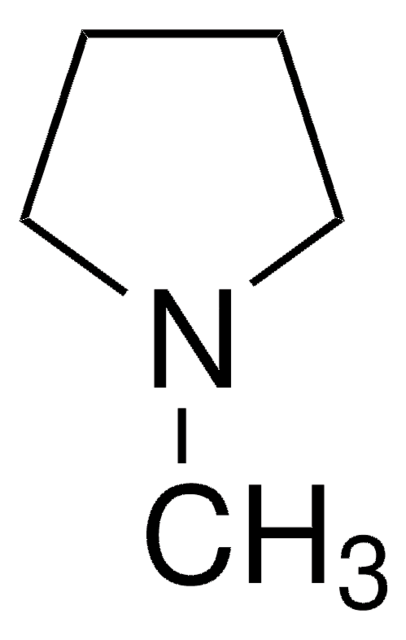
1-Ethylpiperidine
99%
Synonym(s):
N-Ethylpiperidine, NSC 2090
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H15N
CAS Number:
Molecular Weight:
113.20
Beilstein:
102643
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.444 (lit.)
bp
131 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
SMILES string
CCN1CCCCC1
InChI
1S/C7H15N/c1-2-8-6-4-3-5-7-8/h2-7H2,1H3
InChI key
HTLZVHNRZJPSMI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
Synthesis of multiprotected kanosamine
Selective acylation in peptide synthesis
Reagent for:
Stereoselective aldol condensation reactions for synthesis of β-lactam antibiotics
Diastereoselective synthesis of aldols
Crossed Claisen ester condensation
Synthesis of multiprotected kanosamine
Selective acylation in peptide synthesis
Reagent for:
Stereoselective aldol condensation reactions for synthesis of β-lactam antibiotics
Diastereoselective synthesis of aldols
Crossed Claisen ester condensation
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
62.6 °F
Flash Point(C)
17 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoo Tanabe and Teruaki Mukaiyama
Chemistry Letters (Jpn), 11, 1813-1816 (1986)
Laurence Miesch, et al
Synthesis, 1, 161-167 (2011)
Mankil Jung and Marvin J. Miller
Tetrahedron Letters, 26, 977-980 (1985)
Amino-acids and peptides. XXIV. The use of esters of 1-hydroxypiperidine and of other NN-dialkylhydroxylamines in peptide synthesis and as selective acylating agents.
B O Handford et al.
Journal of the Chemical Society. Perkin transactions 1, Dec, 6814-6827 (1965-12-01)
Shigeki Sano et al.
Chemical & pharmaceutical bulletin, 50(9), 1300-1302 (2002-09-19)
Tetrasubstituted (Z)-alkenes were readily prepared through the Horner-Wadsworth-Emmons reactions of methyl 2-[bis(2,2,2-trifluoroethyl)phosphono]propionate with aryl alkyl ketones by employing Sn(OSO(2)CF(3))(2) and N-ethylpiperidine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service