D27004
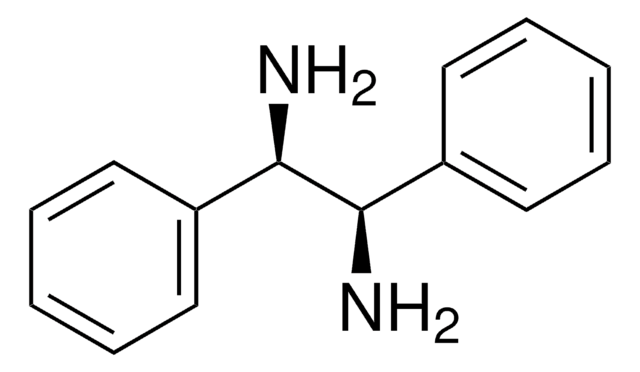
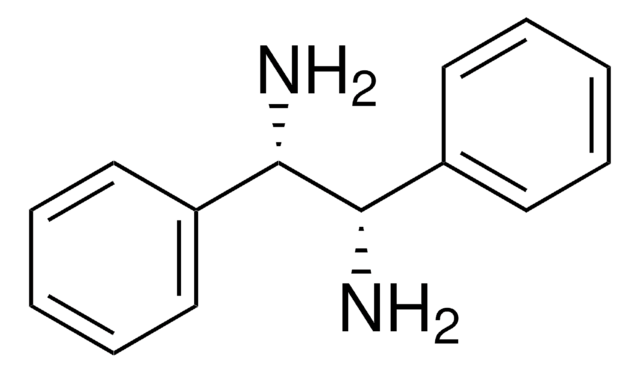
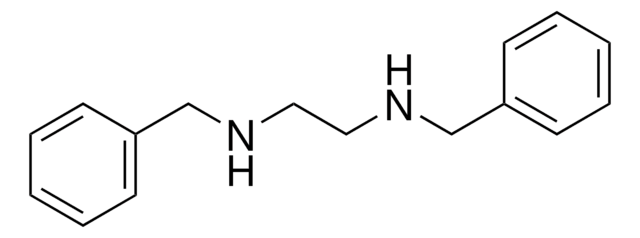
N,N′-Diphenylethylenediamine
98%
Synonym(s):
1,2-Dianilinoethane, N,N′-Ethylenedianiline, Wanzlick’s Reagent for aldehydes
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5NHCH2CH2NHC6H5
CAS Number:
Molecular Weight:
212.29
Beilstein:
646740
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
65-67 °C (lit.)
SMILES string
C(CNc1ccccc1)Nc2ccccc2
InChI
1S/C14H16N2/c1-3-7-13(8-4-1)15-11-12-16-14-9-5-2-6-10-14/h1-10,15-16H,11-12H2
InChI key
NOUUUQMKVOUUNR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N′-Diphenylethylenediamine can be used:
- To prepare nickel(II) chelates to study their chemical reactivities.
- To prepare N-heterocyclic carbene (NHC) adducts by reacting with substituted benzaldehydes.
- As a starting material to prepare substituted cyclic poly(methyl methacrylate)s.
Other Notes
Remainder mainly 1,4-diphenylpiperazine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wen-Juan Wei et al.
Chirality, 22(6), 604-611 (2009-11-10)
L-Dibenzoyl tartaric acid was mono-esterified with benzyl alcohol, and then chlorinated with SOCl(2) to give (2S,3S)-1-(benzyloxy)-4-chloro-1,4-dioxobutane-2,3-diyl dibenzoate (Selector 1). (1R,2R)-1,2-Diphenylethylenediamine was mono-functionalized with phenyl isocyanate and phenylene diisocyanate in sequence to give (1R,2R)-1,2-diphenyl-2-(3-phenylureido)ethyl 4- isocyanatophenylurea (Selector 2). Two brush-type chiral
M Noji et al.
Chemico-biological interactions, 51(1), 37-48 (1984-09-01)
A series of Pt(II) complexes containing 1,2-diphenylethylenediamine (stien) isomers were synthesized and tested for their antitumor activity against leukemia L1210. Among the Pt(II) complexes examined water-soluble Pt(II) complexes with sulfate, nitrate and D-glucuronate ions as leaving groups exhibited relatively high
Yoshitane Imai et al.
Organic letters, 10(3), 469-471 (2008-01-11)
In a two-component columnar host system composed of racemic (rac)-1,2-diphenylethylenediamine and rac-1,1'-binaphthyl-2,2'-dicarboxylic acid, a cavity tuning mechanism resulted from changes in the structure of the columns using a specific combination of the following four molecules: (1R,2R)-1, (1S,2S)-1, (R)-2, and (S)-2.
Determination of plasma catecholamines via condensation with diphenylethylenediamine: simplification of the procedure.
P Husek et al.
Journal of chromatography, 533, 166-170 (1990-11-30)
Yangzhou Li et al.
Organic & biomolecular chemistry, 3(14), 2513-2518 (2005-07-07)
Polymer-supported chiral ligands 9 and 17 were prepared based on Noyori's (1S,2S)- or (1R,2R)-N-(p-tolylsulfonyl)-1,2-diphenylethylenediamine. The combination with [RuCl2(p-cymene)]2 has been shown to exhibit high activities and enantioselectivities for heterogeneous asymmetric transfer hydrogenation of aromatic ketones (19a-c) with formic acid-triethylamine azeotrope
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,2,4]Triazolo[1,5-a][1,3,5]triazin-7-amine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/362/413/8a902135-3f29-47f0-8393-a194caf2c230/640/8a902135-3f29-47f0-8393-a194caf2c230.png)