926175
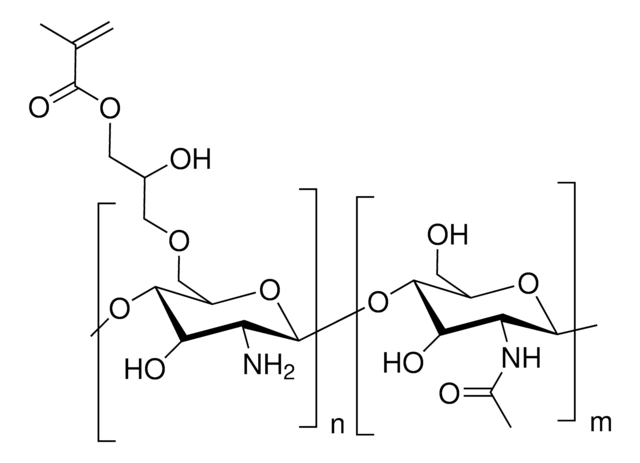
Glycol Chitosan Methacrylate
Degree of methacrylation ∼45%
Synonym(s):
Chitosan hydrogel, Crosslinkble chitosan, Glycol chitosan, Methacrylated glycol chitosan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[C14H25NO8]n [C10H17NO6]m
UNSPSC Code:
12352201
NACRES:
NA.23
Recommended Products
Quality Level
form
powder
color
white to light yellow, light brown
suitability
conforms to structure for NMR
storage temp.
2-8°C
Application
Glycol chitosan is soluble at neutral pH and possesses potentially useful biological properties such as good biocompatibility accelerate wound healing, and antimicrobial properties, and it is less toxic and provide to stimulates chondrocyte growth at low concentrations. Methacrylated glycol chitosan is photo- and thermally cross-linkable and is used as a precursor for the preparation of hydrogels used in biomedical applications including drug delivery, tissue engineering, and 3D bioprinting.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhilong Shi et al.
Biomaterials, 27(11), 2440-2449 (2005-12-13)
Although total joint replacement has become commonplace in recent years, bacterial infection remains a significant complication following this procedure. One approach to reduce the incidence of joint replacement infection is to add antimicrobial agents to the bone cement used to
Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications
Tapan Kumar Giri Amrit Thakur, et al.
Acta Pharmaceutica Sinica. B, Volume 2, Issue 5, 439-449 (2012)
K Y Lee et al.
Biomaterials, 16(16), 1211-1216 (1995-11-01)
Chitosan was selectively N-acylated with various carboxylic anhydrides, e.g., acetic, propionic, n-butyric, n-valeric and n-hexanoic anhydrides, in the presence of methanol. The degree of N-acylation of about 20-50% was obtainable without occurrence of gelation by using carboxylic anhydrides of 0.3-1.2
Brian G Amsden et al.
Biomacromolecules, 8(12), 3758-3766 (2007-11-23)
Glycol chitosan is a derivative of chitosan that is soluble at neutral pH and possesses potentially useful biological properties. With the goal of obtaining biocompatible hydrogels for use as tissue engineering scaffolds or drug delivery depots, glycol chitosan was converted
Evaluation of the Properties of Soluble Chitosan and Chitosan Microspheres
Carreno-Gomez, B.; Duncan, R
International Journal of Pharmaceutics, 148 (2), 231-240 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service