675903
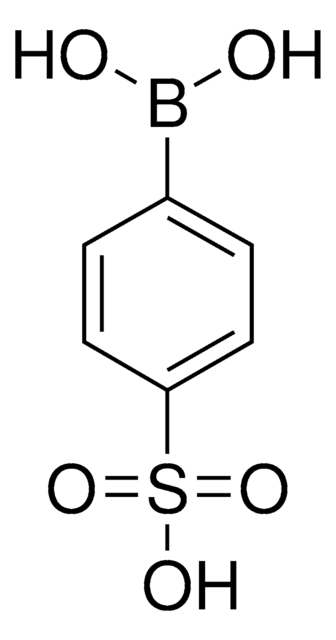
4-(Methanesulfonyl)phenylboronic acid
≥95.0%
Synonym(s):
4-(Methanesulfonyl)benzeneboronic acid, 4-(Methylsulfonyl)phenylboronic acid, 4-Methansulfonylphenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(H3CSO2)C6H4B(OH)2
CAS Number:
Molecular Weight:
200.02
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
mp
289-293 °C
functional group
sulfone
SMILES string
CS(=O)(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H9BO4S/c1-13(11,12)7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
InChI key
VDUKDQTYMWUSAC-UHFFFAOYSA-N
Related Categories
General description
Contains varying amounts of anhydride
Application
4-(Methanesulfonyl)phenylboronic acid may be used as reagent for:
Reagent used in Preparation of
- sequential Suzuki cross-coupling reactions
- Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids
- directed metalation and regioselective functionalization of 3-bromofuran and related heterocycles
- Barton-Zard pyrrole cyclocondensations and Baeyer-Villiger oxidations
- diplar cycloaddition and palladium-catalyzed cross-coupling processes
- continuous flow Suzuki reactions for odanacatib intermediate synthesis
Reagent used in Preparation of
- diarylaminopyridines as potential anti-malarial agents
- hydropyranopyrazine via chloropyrazinecarboxaldehyde and olefination
- biaryl sulfone derivatives as antagonists of the histamine H3 receptor
- novel kinase inhibitor scaffolds with potential antitumor effects
- Hepatitis C virus inhibition activity of N-hydroxyisoquinoline di
Highly effective boronic acid used in a rhodium-catalyzed asymmetric 1,4-addition to 4-oxobutenamides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jamie L Zigterman et al.
The Journal of organic chemistry, 72(23), 8870-8876 (2007-10-12)
A variety of 4-oxobutenamides 1 were subjected to rhodium-catalyzed conjugate addition with arylboronic acids providing high regio- and enantioselectivity (97:3 to >99:1, >96% ee) and moderate to excellent yields (54-99%). The key to high selectivity is the use of sterically
Combined batch and continuous flow procedure to the chemo-enzymatic synthesis of biaryl moiety of Odanacatib.
de Oliveira Lopes R, et al.
Journal of Molecular Catalysis. B, Enzymatic, 104, 101-107 (2014)
Pamela Kassis et al.
European journal of medicinal chemistry, 46(11), 5416-5434 (2011-09-29)
We here report the synthesis and biological evaluation of new 3-[(2-indolyl)]-5-phenyl-3,5-pyridine, 3-[(2-indolyl)]-5-phenyl-2,4-pyridine and 3-[(2-indolyl)]-5-phenyl-2,6-pyrazine derivatives designed as potential CDK inhibitors. Indoles and phenyls were used to generate several substitutions of the pyridine and pyrazine rings. The synthesis included Stille or
Optimization of a novel kinase inhibitor scaffold for the dual inhibition of JAK2 and FAK kinases
Zificsak, C. A.; et al.
Bioorganic & Medicinal Chemistry, 22, 133-137 (2012)
Facile Access to 3,5-Dihalogenated Pyrazoles by Sydnone Cycloaddition and their Versatile Functionalization by Pd-Catalyzed Cross-Coupling Processes
Delaunay, T.; et al.
European Journal of Medicinal Chemistry, 20-21, 3837-3848 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service