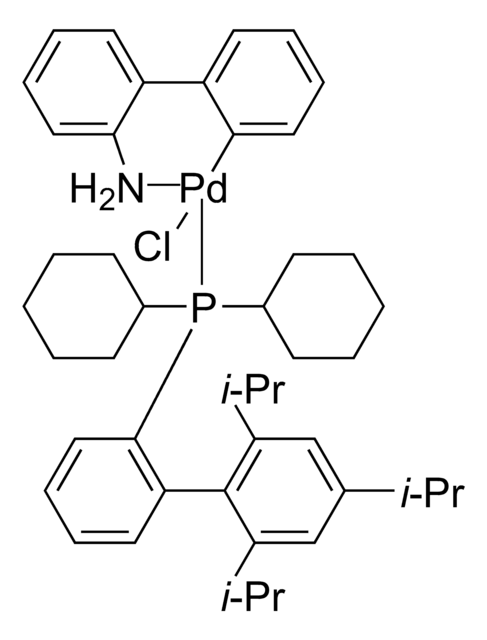
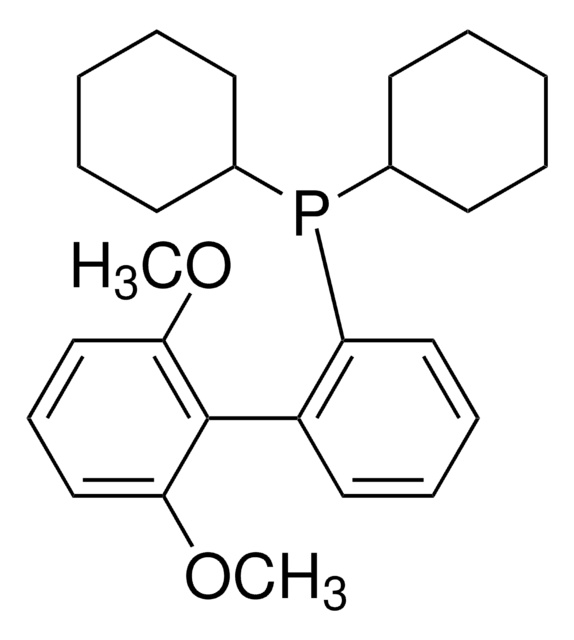
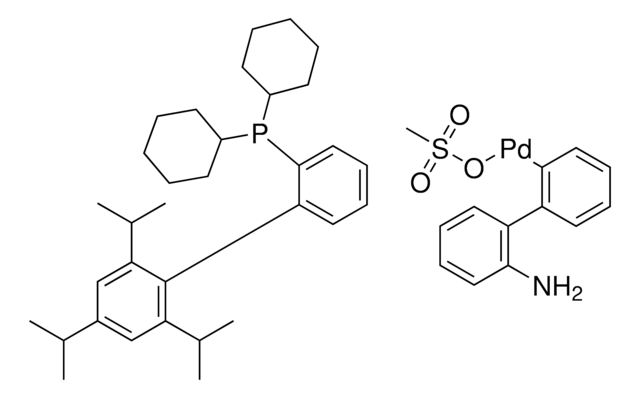
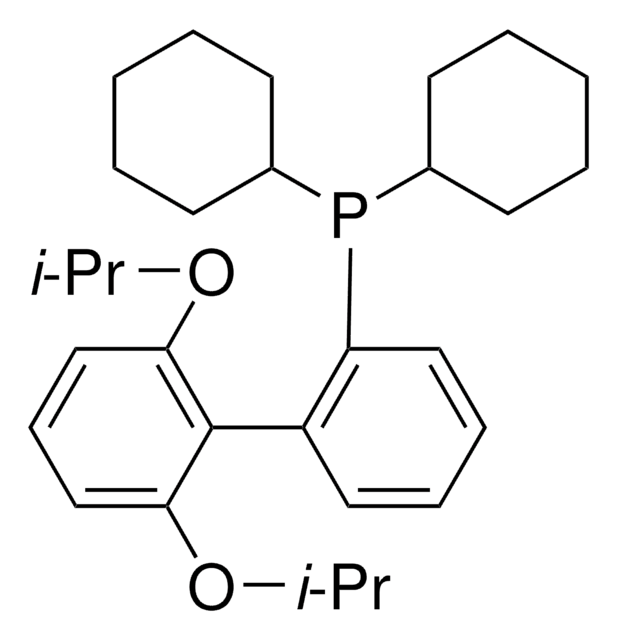
638064
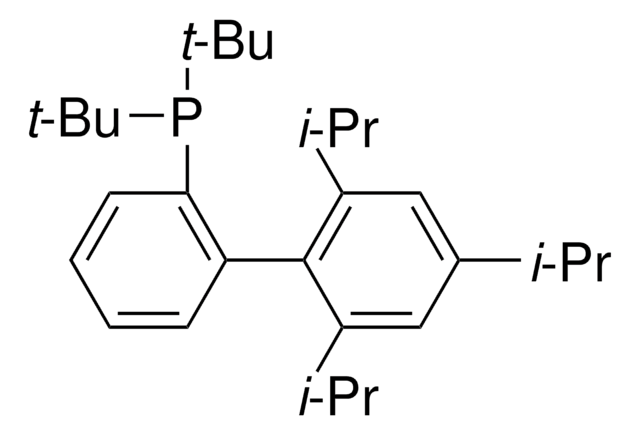
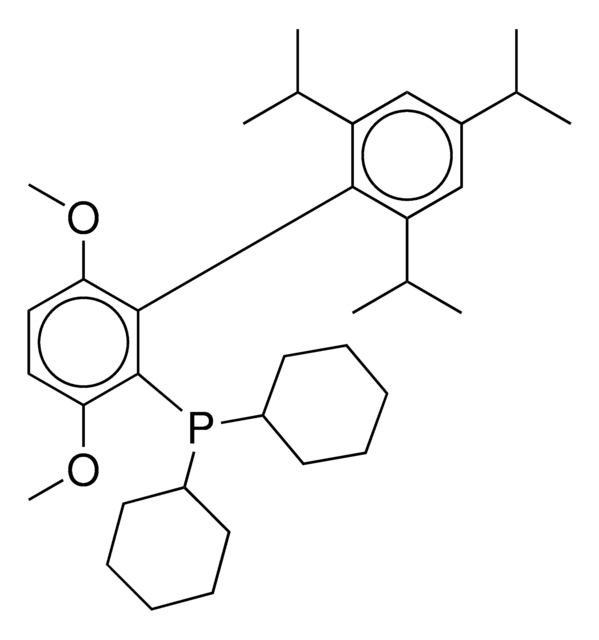
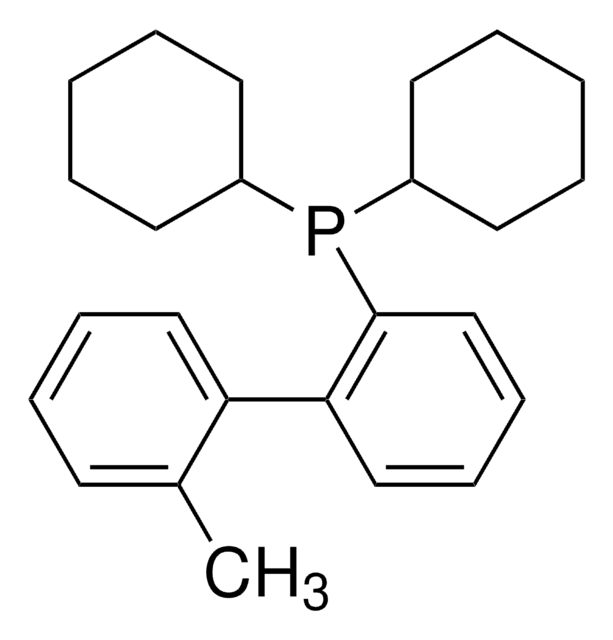
XPhos
98%
Synonym(s):
2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl
About This Item
Recommended Products
Quality Level
Assay
98%
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Hiyama Coupling
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
greener alternative product score
old score: 2
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Atom Economy
Use of Renewable Feedstocks
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-190 °C (lit.)
functional group
phosphine
greener alternative category
SMILES string
CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C2=C(P(C3CCCCC3)C4CCCCC4)C=CC=C2
InChI
1S/C33H49P/c1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28/h13-14,19-25,27-28H,7-12,15-18H2,1-6H3
InChI key
UGOMMVLRQDMAQQ-UHFFFAOYSA-N
General description
Application
Direct annulation of 2-haloanilines to indoles and tryptophans catalyzed by Pd. Synthesis of regioregular polythiophenes.
For small scale and high throughput uses, product is also available as ChemBeads (928364)
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
- Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides.
- Along with pre-milled palladium(II) acetate as a pre-catalyst for the Stille cross-coupling of aryl chlorides with tributylarylstannanes to form the corresponding biaryl compounds.
- Along with platinum chloride to catalyze the hydrosilylation of terminal arylalkynes with silanes to form functionalized β-(E)-vinylsilanes.
Legal Information
related product
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A variety of palladium-catalyzed cross-coupling reactions can be run under mild room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
Buchwald Phosphine Ligands
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service