531197
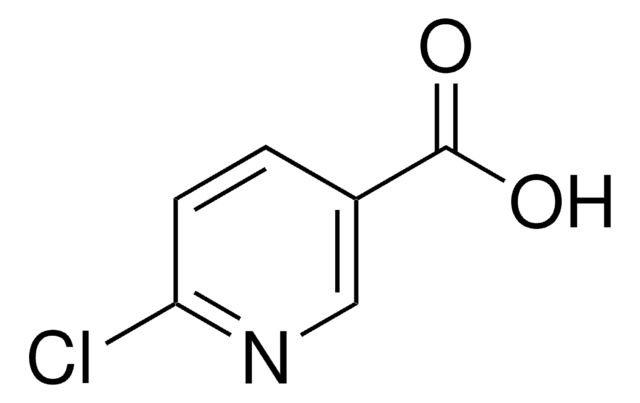
Ethyl 6-chloropyridine-3-carboxylate
97%
Synonym(s):
Ethyl 6-chloronicotinate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8ClNO2
CAS Number:
Molecular Weight:
185.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
26-30 °C (lit.)
functional group
chloro
ester
SMILES string
CCOC(=O)c1ccc(Cl)nc1
InChI
1S/C8H8ClNO2/c1-2-12-8(11)6-3-4-7(9)10-5-6/h3-5H,2H2,1H3
InChI key
ILDJJTQWIZLGPO-UHFFFAOYSA-N
General description
Ethyl 6-chloropyridine-3-carboxylate (Ethyl-6-chloronicotinate, E-6-ClN) undergoes direct amidation on reacting with benzylamine in the presence lanthanum trifluoromethanesulfonate La(OTf)3. The structural and physicochemical properties of E-6-ClN have been investigated based on its spectroscopic data, time-dependent density functional theory and density of state diagrams.
Application
Ethyl 6-chloropyridine-3-carboxylate (Ethyl 6-chloronicotinate) may be used in the preparation of ethyl 5-{[5-(1H-benzimidazol-2-yl)pyridin-2-yl]ethynyl}pyridine-2-carboxylate by reacting with 2-[6-(ethynyl)pyridin-3-yl]-1H-benzimidazole under microwave irradiation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The spectroscopic (FT-IR, FT-Raman, dispersive Raman and NMR) study of ethyl-6-chloronicotinate molecule by combined density functional theory.
Karabacak M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 153, 754-770 (2016)
Microwave-assisted Sonogashira coupling of novel 2-[6-(arylethynyl) pyridin-3-yl]-1H-benzimidazole derivatives.
Raut CN, et al.
ARKIVOC (Gainesville, FL, United States), 11, 105-114 (2009)
Hiroyuki Morimoto et al.
Organic letters, 16(7), 2018-2021 (2014-03-26)
Lanthanum trifluoromethanesulfonate is an effective single-component catalyst for synthesizing a variety of amides directly from esters and amines under mild conditions. Highly selective amidation of esters and amines, as well as catalyst-controlled amidation of esters, demonstrated the effectiveness of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service