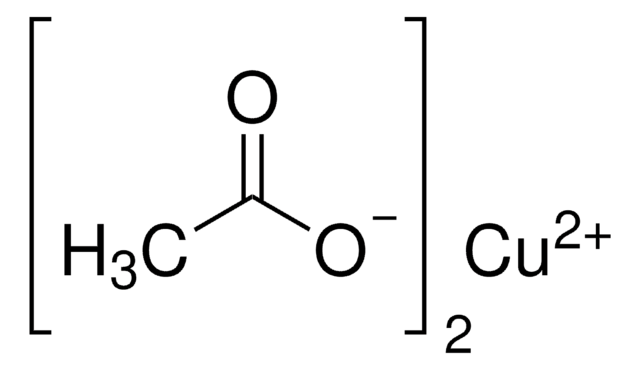
441627
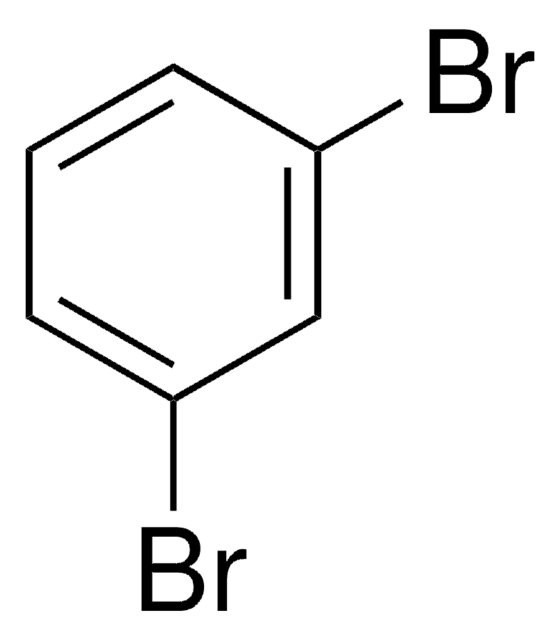
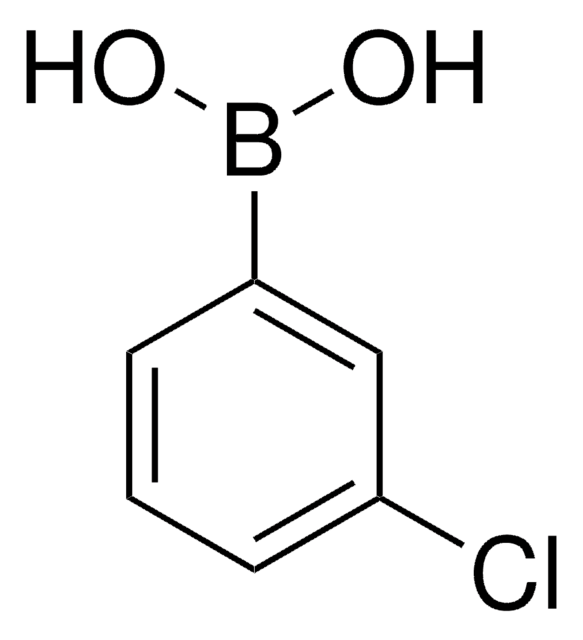
3-Bromophenylboronic acid
≥95%
Synonym(s):
3-Bromobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4B(OH)2
CAS Number:
Molecular Weight:
200.83
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder or crystals
mp
164-168 °C (lit.)
functional group
bromo
SMILES string
OB(O)c1cccc(Br)c1
InChI
1S/C6H6BBrO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4,9-10H
InChI key
AFSSVCNPDKKSRR-UHFFFAOYSA-N
Related Categories
Application
Reactant involved in a variety of organic reactions including:
- Oxidative cross coupling
- Gold salt catalyzed homocoupling
- 1,4-Addition reactions with α,β-unsaturated ketones
- Enantioselective addition reactions
- Suzuki-Miyaura coupling for synthesis of anthranilamide-protected arylboronic acids
- C-H Functionalization of quinones
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G K Surya Prakash et al.
Organic letters, 6(13), 2205-2207 (2004-06-18)
[reaction: see text] A mixture of nitrate salt and chlorotrimethylsilane is found to be an efficient regioselective nitrating agent for the ipso-nitration of arylboronic acids to produce the corresponding nitroarenes in moderate to excellent yields. High selectivity, simplicity, and convenience
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 441627-1G | |
| 441627-5G | 4061832904504 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)