334251
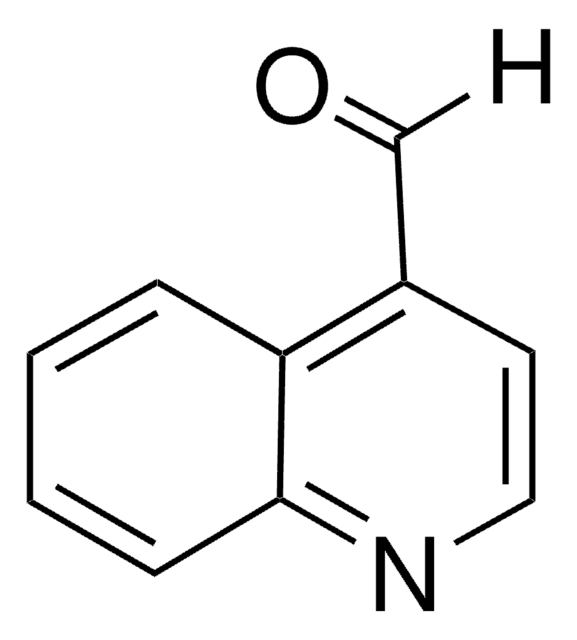
2-Quinolinecarboxaldehyde
97%
Synonym(s):
2-Formylquinoline, 2-Quinolinecarbaldehyde, 2-Quinolylaldehyde, 2-Quinolylcarbaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H7NO
CAS Number:
Molecular Weight:
157.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
70-72 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO/c12-7-9-6-5-8-3-1-2-4-10(8)11-9/h1-7H
InChI key
WPYJKGWLDJECQD-UHFFFAOYSA-N
Application
2-Quinolinecarboxaldehyde was used in the preparation of:
- 3-(2-quinolyl)-1-phenyl-2-propenone via rapid, tandem aldol-Michael reactions with the lithium, sodium and potassium enolates of acetophenone
- imine-type ligands
- sugar-quinoline fluorescent sensor for the detection of Hg2+ in natural water
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper (I)-imine complexes: Synthesis and catalytic activity in olefin cyclopropanation
Attilio Ardizzoia G, et al.
Inorgorganica Chimica Acta, 362(10), 3507-3512 (2009)
Addressing the unusual reactivity of 2-pyridinecarboxaldehyde and 2-quinolinecarboxaldehyde in base-catalyzed aldol reactions with acetophenone.
Wachter-Jurcsak N, et al.
Tetrahedron Letters, 39(23), 3903-3906 (1998)
Shengju Ou et al.
Chemical communications (Cambridge, England), (42)(42), 4392-4394 (2006-10-24)
A selective and sensitive fluorescent sensor for detection of Hg2+ in natural water was achieved by incorporating the well-known fluorophore quinoline group and a water-soluble D-glucosamine group within one molecule.
Prinessa Chellan et al.
Chemistry (Weinheim an der Bergstrasse, Germany) (2018-04-14)
Fourteen novel arene RuII , and cyclopentadienyl (Cpx ) RhIII and IrIII complexes containing an N,N'-chelated pyridylimino- or quinolylimino ligand functionalized with the antimalarial drug sulfadoxine have been synthesized and characterized, including three by X-ray crystallography. The rhodium and iridium
Diana Costa et al.
Biomacromolecules, 18(9), 2928-2936 (2017-08-17)
The development of a suitable delivery system and the targeting of intracellular organelles are both essential for the success of drug and gene therapies. The conception of fluorescent ligands, displaying targeting specificity together with low toxicity, is an emerging and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service