259276
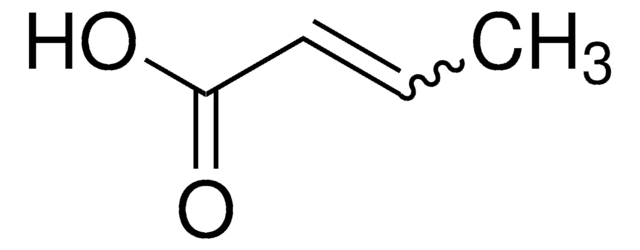
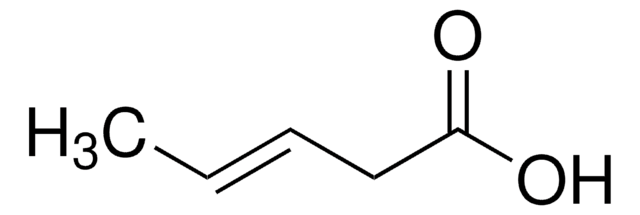
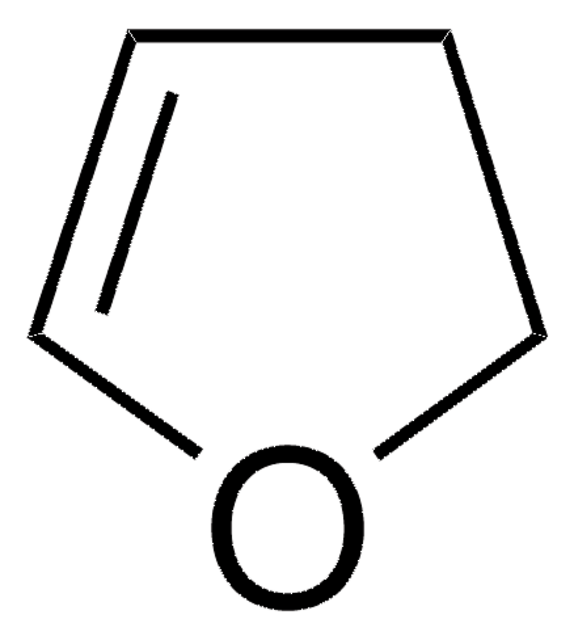
2-Pentenoic acid
predominantly trans, 98%
Synonym(s):
trans-2-Pentenoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5CH=CHCO2H
CAS Number:
Molecular Weight:
100.12
Beilstein:
1720312
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
impurities
10% cis isomer (maximum)
refractive index
n20/D 1.452 (lit.)
bp
106 °C/20 mmHg (lit.)
mp
9-11 °C (lit.)
density
0.99 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
[H]\C(CC)=C(\[H])C(O)=O
InChI
1S/C5H8O2/c1-2-3-4-5(6)7/h3-4H,2H2,1H3,(H,6,7)/b4-3+
InChI key
YIYBQIKDCADOSF-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Pentenoic acid has been used in preparation of new nonsteroidal human androgen receptor (hAR) agonists from an hAR antagonist pharmacophore, 2(1H)-piperidino[3,2-g]quinolinone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Zhi et al.
Bioorganic & medicinal chemistry letters, 9(7), 1009-1012 (1999-05-07)
New nonsteroidal human androgen receptor (hAR) agonists were developed from an hAR antagonist pharmacophore, 2(1H)-piperidino[3,2-g]quinolinone. (+/-)-trans-7,8-Diethyl-4-trifluoromethyl-2(H)-piperidino-[3,2-g]quinoli none was synthesized and demonstrated potent hAR agonist activity (EC50=3 nM) in the cell-based cotransfection assay and high binding affinity (Ki=16 nM) in the
Norlaily Ahmad et al.
Biomacromolecules, 20(7), 2506-2514 (2019-06-28)
Inflammatory conditions are frequently accompanied by increased levels of active proteases, and there is rising interest in methods for their detection to monitor inflammation in a point of care setting. In this work, new sensor materials for disposable single-step protease
Gilles Brackman et al.
PloS one, 6(1), e16084-e16084 (2011-01-21)
Many bacteria, including Vibrio spp., regulate virulence gene expression in a cell-density dependent way through a communication process termed quorum sensing (QS). Hence, interfering with QS could be a valuable novel antipathogenic strategy. Cinnamaldehyde has previously been shown to inhibit
Joachim Morrens et al.
Neuron, 106(1), 142-153 (2020-02-07)
Dopamine neurons mediate the association of conditioned stimuli (CS) with reward (unconditioned stimuli, US) by signaling the discrepancy between predicted and actual reward during the US. Some theoretical models suggest that learning is also influenced by the salience or associability
J P D van Veldhoven et al.
Bioorganic & medicinal chemistry letters, 21(9), 2736-2739 (2010-12-21)
Nicotinic acid (niacin) has been used for decades as an antidyslipidemic drug in man. Its main target is the hydroxy-carboxylic acid receptor HCA2 (GPR109A), a G protein-coupled receptor. Other acids and esters such as methyl fumarate also interact with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service