All Photos(1)
About This Item
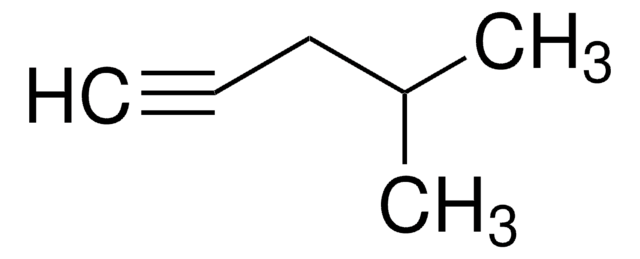
Linear Formula:
CH3CH2CH2C≡CH
CAS Number:
Molecular Weight:
68.12
Beilstein:
1697133
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
6.8 psi ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.385 (lit.)
bp
40 °C (lit.)
mp
−106-−105 °C (lit.)
density
0.691 g/mL at 25 °C (lit.)
SMILES string
CCCC#C
InChI
1S/C5H8/c1-3-5-4-2/h1H,4-5H2,2H3
InChI key
IBXNCJKFFQIKKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Selective and non-selective hydrogenation of 1-pentyne catalyzed by silica-supported palladium has been studied by in situ X-ray absorption spectroscopy.
Application
1-Pentyne has been used in preparation of:
- lithium acetylides, required for asymmetric synthesis of α,α-dibranched propargyl sulfinamides
- 7-hydroxy-10-methoxy-3H-naphtho[2.1-b]pyrans
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-4.0 °F - closed cup
Flash Point(C)
-20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manish Rawat et al.
Journal of the American Chemical Society, 128(34), 11044-11053 (2006-08-24)
The carbene complex 5-(2,2-dimethyl-2H-chromene)methoxylmethylene chromium pentacarbonyl will undergo a benzannulation reaction with phenylacetylene, 1-pentyne, 3-hexyne, and trimethylsilylacetylene to give 7-hydroxy-10-methoxy-3H-naphtho[2.1-b]pyrans as the primary product. These compounds are difficult to obtain pure due to their sensitivity to air. If the benzannulation
Andrew W Patterson et al.
The Journal of organic chemistry, 71(18), 7110-7112 (2006-08-26)
Addition of lithium acetylides prepared from 1-pentyne, phenylacetylene, and trimethylsilylacetylene to diverse N-tert-butanesulfinyl ketimines affords a range of alpha,alpha-dibranched propargyl sulfinamides in generally good yields (up to 87%) and with high diastereoselectivities (up to >99:1). Acidic cleavage of the tert-butanesulfinyl
Min Wei Tew et al.
Physical chemistry chemical physics : PCCP, 14(16), 5761-5768 (2012-03-17)
The catalytically active phase of silica-supported palladium catalysts in the selective and non-selective hydrogenation of 1-pentyne was determined using in situ X-ray absorption spectroscopy at the Pd K and L(3) edges. Upon exposure to alkyne, a palladium carbide-like phase rapidly
Quan-De Wang et al.
International journal of molecular sciences, 20(13) (2019-07-03)
Hydrogen atom abstraction from propargyl C-H sites of alkynes plays a critical role in determining the reactivity of alkyne molecules and understanding the formation of soot precursors. This work reports a systematic theoretical study on the reaction mechanisms and rate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service