238112
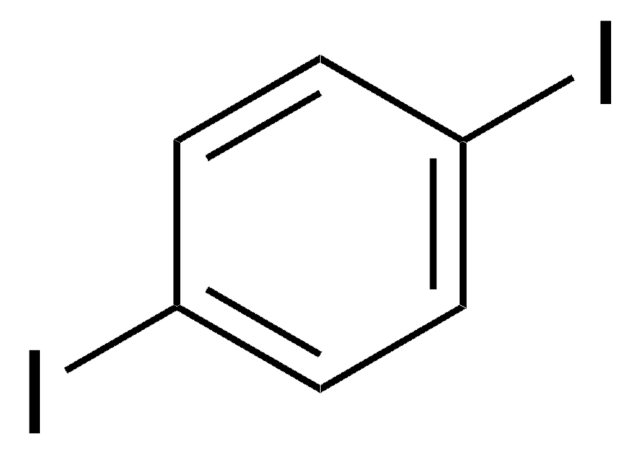
1,2-Diiodobenzene
98%
Synonym(s):
1,2-Phenylene diiodide, o-Diiodobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4I2
CAS Number:
Molecular Weight:
329.90
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.718 (lit.)
bp
152 °C/15 mmHg (lit.)
density
2.524 g/mL at 25 °C (lit.)
functional group
iodo
storage temp.
2-8°C
SMILES string
Ic1ccccc1I
InChI
1S/C6H4I2/c7-5-3-1-2-4-6(5)8/h1-4H
InChI key
BBOLNFYSRZVALD-UHFFFAOYSA-N
General description
Photolysis of 1,2-diiodobenzene in cyclohexane, furan, benzene and benzene containing tetraphenylcyclopentadienone has been studied.
Application
1,2-Diiodobenzene has been used in the preparation of purino[8,9-f]phenanthridines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Photolysis of 1, 2-Diiodobenzene: A Photochemical Source of Benzyne.
Kampmeier JA and Hoffmeister E.
Journal of the American Chemical Society, 84(19), 3787-3788 (1962)
Igor Cerna et al.
The Journal of organic chemistry, 75(7), 2302-2308 (2010-03-05)
Intramolecular C-H arylations were employed as a key step in the synthesis of hitherto unknown fused purine systems: 13-substituted purino[8,9-f]phenanthridines and 11-substituted 5,6-dihydropurino[8,9-a]isoquinolines. The purino[8,9-f]phenanthridines were prepared in moderate yields by double C-H arylations of 9-phenylpurines with 1,2-diiodobenzene or, more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service