196819
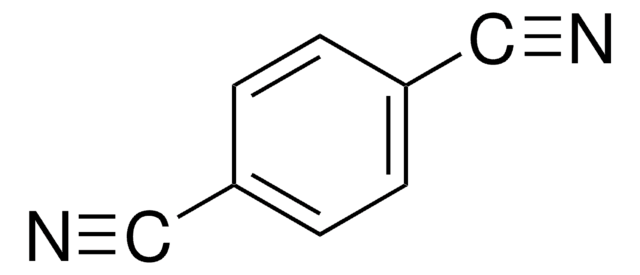
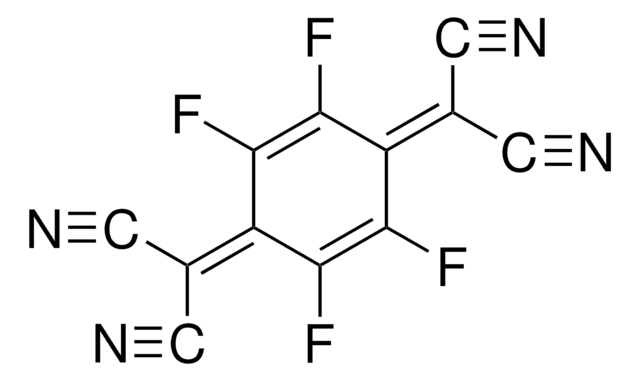
Tetrafluorophthalonitrile
95%
Synonym(s):
1,2-Dicyano-3,4,5,6-tetrafluorobenzene, 3,4,5,6-Tetrafluoro-1,2-benzenedicarbonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6F4(CN)2
CAS Number:
Molecular Weight:
200.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
81-86 °C (lit.)
functional group
fluoro
nitrile
SMILES string
Fc1c(F)c(F)c(C#N)c(C#N)c1F
InChI
1S/C8F4N2/c9-5-3(1-13)4(2-14)6(10)8(12)7(5)11
InChI key
OFLRJMBSWDXSPG-UHFFFAOYSA-N
General description
Tetrafluorophthalonitrile reacts with:
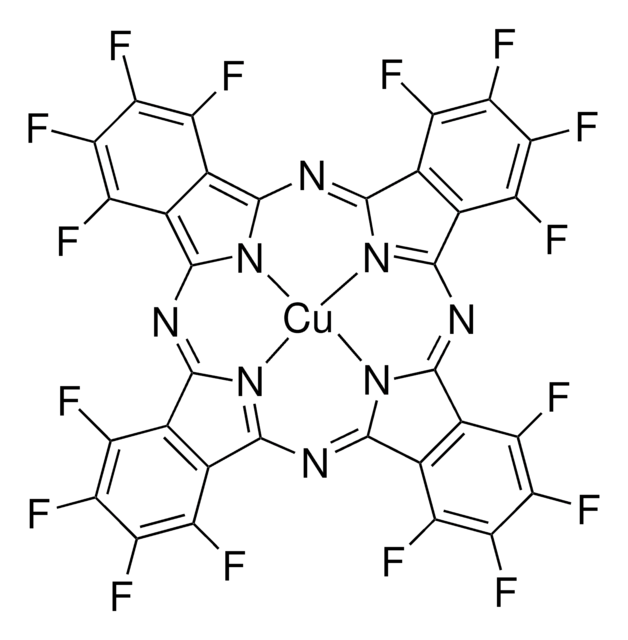
- copper, copper (I) chloride or copper (II) chloride to yield copper (II) hexadecafluorophthalocyanine
- potassium salt of 2-hydroxyhexafluoro-2-propylbenzene to yield 2-phenyl-2-(3,4-dicyano- trifluorophenoxy) hexafluoropropane
- dipotassium salt of 1,3-bis(2-hdroxyhexafluoro-2- propyl) benzene to yield fluorinated phthalonitrile resins
Application
Tetrafluorophthalonitrile was used in the synthesis of dichloro-subphthalocyanine dimers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis of highly fluorinated phthalonitrile resins and cure studies.
Keller TM and Griffith JR.
Journal of Fluorine Chemistry, 13(4), 315-324 (1979)
Synthesis, separation, and characterization of the topoisomers of fused bicyclic subphthalocyanine dimers.
Christian G Claessens et al.
Angewandte Chemie (International ed. in English), 41(14), 2561-2565 (2002-08-31)
Polyfluoroarenes. Part XI. Reactions of tetrafluorophthalonitrile with nucleophilic reagents.
Birchall JM, et al.
J. Chem. Soc. Sect. C, 3, 456-462 (1970)
Dongkun Yu et al.
The journal of physical chemistry. B, 123(23), 4958-4966 (2019-05-24)
The concept of eutectic molecular liquids (EMLs) was defined, and a strategy to form EMLs based on noncovalent interactions was proposed. We verified the formation and investigated the properties, interaction sites, and interaction energies of the obtained 16 EMLs. Moreover
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service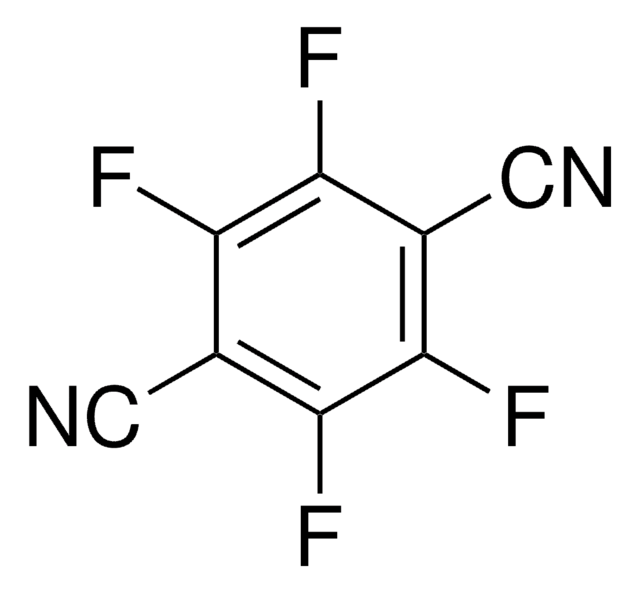
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)