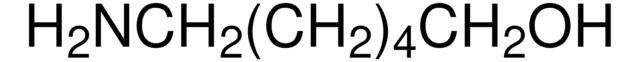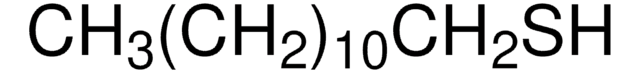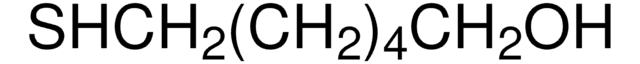178330
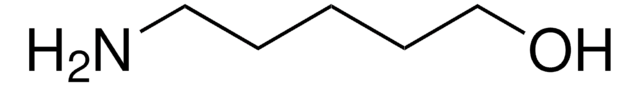
4-Amino-1-butanol
98%
Synonym(s):
4-Hydroxybutylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2N(CH2)4OH
CAS Number:
Molecular Weight:
89.14
Beilstein:
1731411
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
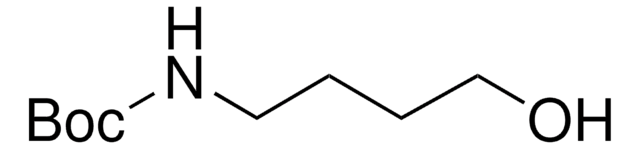
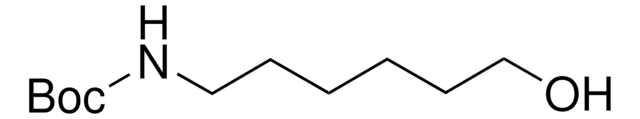
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D ~1.462 (lit.)
bp
206 °C (lit.)
mp
16-18 °C (lit.)
density
0.967 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
NCCCCO
InChI
1S/C4H11NO/c5-3-1-2-4-6/h6H,1-5H2
InChI key
BLFRQYKZFKYQLO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Amino-1-butanol was used in the synthesis of cyclic amines.
4-Amino-1-butanol was used:
- As a linker in the synthesis of highly branched poly(β-amino esters)(HPAEs) for gene delivery.
- As a side chain to modulate antimicrobial and hemolytic activities of copolymers.
- In the total synthesis of (+)-fawcettimine,(+)-fawcettidine, and (−)-lycojapodine A.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Physicochemical and biological evaluation of siRNA polyplexes based on PEGylated Poly(amido amine)s.
Pieter Vader et al.
Pharmaceutical research, 29(2), 352-361 (2011-08-13)
Use of RNA interference as novel therapeutic strategy is hampered by inefficient delivery of its mediator, siRNA, to target cells. Cationic polymers have been thoroughly investigated for this purpose but often display unfavorable characteristics for systemic administration, such as interactions
Collective synthesis of lycopodium alkaloids and tautomer locking strategy for the total synthesis of (−)-lycojapodine A.
Li H, et al.
The Journal of Organic Chemistry, 78(3), 800-821 (2012)
Total Syntheses of Lycopodium Alkaloids (+)?Fawcettimine,(+)?Fawcettidine, and (−)?8?Deoxyserratinine.
Li H, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 124(2), 506-510 (2012)
Cationic spacer arm design strategy for control of antimicrobial activity and conformation of amphiphilic methacrylate random copolymers.
Palermo E F, et al.
Biomacromolecules, 13(5), 1632-1641 (2012)
Synthesis of cyclic amines and their alkyl derivatives from amino alcohols over supported copper catalysts.
Applied Catalysis, 53(1), 107-115 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service