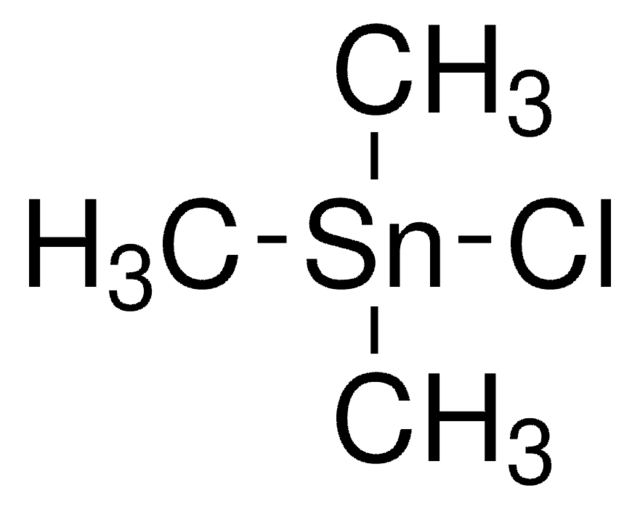146498
Trimethyltin chloride
Synonym(s):
Chlorotrimethylstannane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3SnCl
CAS Number:
Molecular Weight:
199.27
Beilstein:
3535111
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
crystals
Quality Level
mp
37-39 °C (lit.)
SMILES string
C[Sn](C)(C)Cl
InChI
1S/3CH3.ClH.Sn/h3*1H3;1H;/q;;;;+1/p-1
InChI key
KWTSZCJMWHGPOS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Trimethyltin chloride is an organotin reagent widely used in transferring trimethylstannyl groups onto the substrates to synthesize various organostannanes. Trimethylstannyl compounds derived from this reagent, are extensively used in the palladium-catalyzed Stille coupling reactions.
Application
Trimethyltin chloride can be used as a precursor to synthesize trimethyltin hydride, cyanide, methoxide, azide, and lithium compounds.
It can also be used as a reagent to prepare:
Me3SnCl can also be used as a Lewis acid catalyst in asymmetric allylic alkylation reactions.
It can also be used as a reagent to prepare:
- Organotrimethyltin derivatives by reacting with organocopper compounds via transmetalation reaction.
- Acetophenone by palladium-catalyzed coupling reaction with benzoyl chloride.
- Optically active propargyl trimethylstannane by treating with chiral allenyltitanium.
- Trimethylstannyl nucleophiles, which are applicable in the formation of Sn-C bonds via SN2 reactions, SRN1 reactions, and halogen-metal exchanges.
- Carbocycles by reacting with unactivated dienes or trienes via radical-mediated carbocyclization reaction in the presence of NaBH3CN and a catalytic amount of AIBN.
Me3SnCl can also be used as a Lewis acid catalyst in asymmetric allylic alkylation reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chlorotrimethylstannane.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
Chlorotrimethylstannane
Yoshinori Yamamoto, et al.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2007)
Replacing alkoxy groups with alkylthienyl groups: a feasible approach to improve the properties of photovoltaic polymers.
Huo H, et al.
Angewandte Chemie (International Edition in English), 50(41), 9697-9702 (2011)
Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism.
Milstein D and Stille J K
Journal of the American Chemical Society, 101(17), 4992-4998 (1979)
Yu Xi et al.
Journal of pineal research, 67(3), e12596-e12596 (2019-07-25)
Trimethyltin chloride (TMT) is a potent neurotoxin that causes neuroinflammation and neuronal cell death. Melatonin is a well-known anti-inflammatory agent with significant neuroprotective activity. Male C57BL/6J mice were intraperitoneally injected with a single dose of melatonin (10 mg/kg) before exposure to TMT (2.8 mg/kg, ip). Thereafter, the mice received
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service