All Photos(1)
About This Item
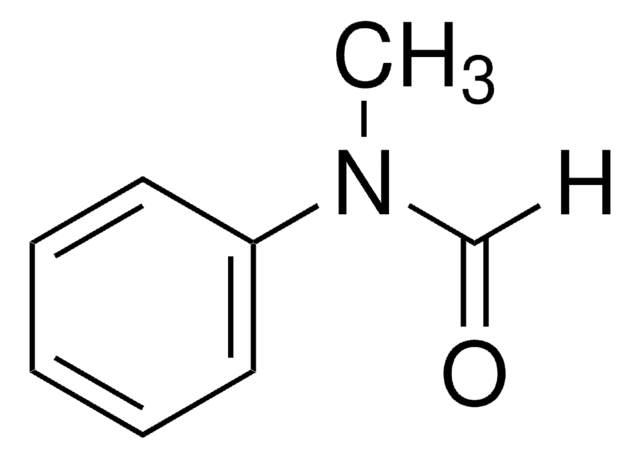
Linear Formula:
C6H5NHCHO
CAS Number:
Molecular Weight:
121.14
Beilstein:
906934
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
bp
166 °C/14 mmHg (lit.)
mp
46-48 °C (lit.)
solubility
water: soluble 25.4 g/L at 20 °C
water: soluble 28.6 g/L at 25 °C
density
1.144 g/mL at 25 °C (lit.)
SMILES string
O=CNc1ccccc1
InChI
1S/C7H7NO/c9-6-8-7-4-2-1-3-5-7/h1-6H,(H,8,9)
InChI key
DYDNPESBYVVLBO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Formanilide was used to study the zero electron kinetic energy(ZEKE) spectra of cis- and trans-formanilide. It was used to investigate the gas-phase structures of the two isomers of the trans-formanilide-water complex by two-colour (1+1′) resonance enhanced multiphoton ionisation (REMPI) and ZEKE spectroscopy.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hydration of a cationic amide group: a ZEKE spectroscopic study of trans-formanilide-H2O.
Ullrich S, et al.
Physical Chemistry Chemical Physics, 4(13), 2897-2903 (2002)
ZEKE photoelectron spectroscopy of the cis and trans isomers of formanilide.
Susanne Ullrich et al.
Angewandte Chemie (International ed. in English), 41(1), 166-168 (2002-12-20)
Aminolysis and hydrolysis of formanilide in water solutions. V. Influence of the substituent in para-position.
B Bergstrand
Acta pharmaceutica Suecica, 22(1), 1-16 (1985-01-01)
Qin Wu et al.
The journal of physical chemistry. A, 110(29), 9212-9218 (2006-07-21)
It is shown that constrained density functional theory (DFT) can be used to access diabatic potential energy surfaces in the Marcus theory of electron transfer, thus providing a means to directly calculate the driving force and the inner-sphere reorganization energy.
Xue-Bo Chen et al.
Journal of the American Chemical Society, 126(29), 8976-8980 (2004-07-22)
In the present study, the five lowest electronic states that control the UV photodissociation of formanilide and benzamide have been characterized using the complete active space self-consistent field theory. The mechanisms for the initial relaxation and subsequent dissociation processes have
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service