All Photos(2)
About This Item
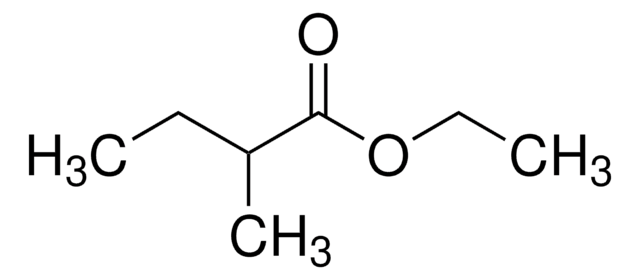
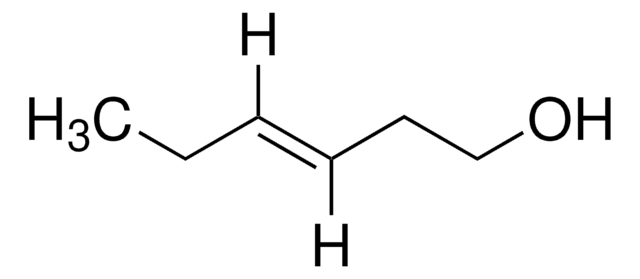
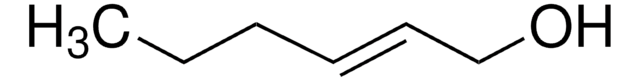
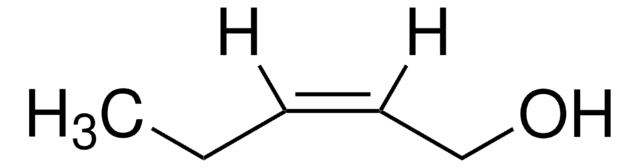
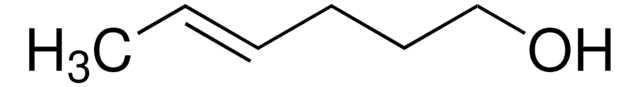
Linear Formula:
CH3CH2CH2CH=CHCH2OH
CAS Number:
Molecular Weight:
100.16
Beilstein:
1719709
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.438 (lit.)
bp
158-160 °C (lit.)
density
0.849 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(CO)=C(\[H])CCC
InChI
1S/C6H12O/c1-2-3-4-5-6-7/h4-5,7H,2-3,6H2,1H3/b5-4+
InChI key
ZCHHRLHTBGRGOT-SNAWJCMRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
trans-2-Hexen-1-ol undergoes allylic epoxidation to yield (2R,3R)-(+)-3-propyloxiranemethanol in high pressure carbon dioxide.
Application
trans-2-Hexen-1-ol was used to evaluate the quality of protected designation of virgin olive oils by headspace solid-phase microextraction-gas chromatography using flame ionization detection and multivariate analysis. It was also used in encapsulation of vanadium catalysts in inorganic and hybrid matrices using sol-gel method.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Multicomponent phase equilibrium measurements and modeling for the allylic epoxidation of trans-2-hexen-1-ol to (2R, 3R)-(+)-3-propyloxiranemethanol in high-pressure carbon dioxide.
Stradi BA, et al.
Journal of Supercritical Fluids, 20(1), 1-13 (2001)
Encapsulation of vanadium complexes in inorganic or hybrid matrices via the sol-gel method: application to the epoxidation of allylic alcohols.
Pellegrino RB and Buffon R.
Journal of the Brazilian Chemical Society, 15(4), 527-531 (2004)
Benoit Berlioz et al.
Journal of agricultural and food chemistry, 54(26), 10092-10101 (2006-12-21)
Headspace solid-phase microextraction (HS-SPME) -gas chromatography using flame ionization detection and multivariate analysis were applied to the study of the specificity of protected designation of origin (PDO) virgin olive oils produced in a southern French region (Alpes-Maritimes) based on their
Leandro Dias Araujo et al.
Food research international (Ottawa, Ont.), 98, 79-86 (2017-06-15)
Elemental sulfur is a fungicide traditionally used to control Powdery Mildew in the production of grapes. The presence of sulfur residues in grape juice has been associated with increased production of hydrogen sulfide during fermentation, which could take part in
Xiaotong Lyu et al.
Food chemistry, 346, 128914-128914 (2021-01-09)
The antioxidants sulfur dioxide (50 ppm) and ascorbic acid (100 ppm) were added to grapes soon after harvest at crushing. The chemical composition and sensory profile of Sauvignon Blanc, Pinot Gris and Chardonnay wines were examined, made from grapes collected at three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service