130648
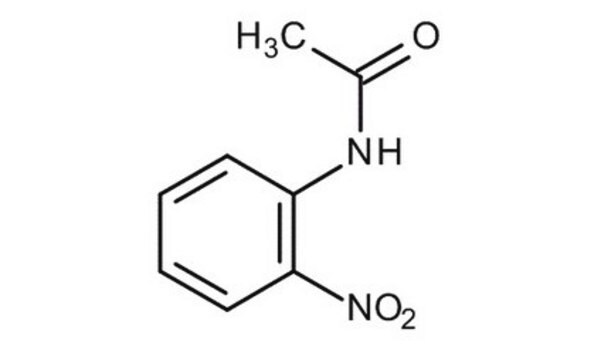
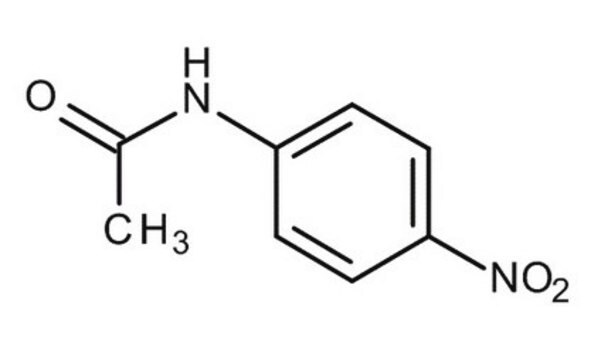
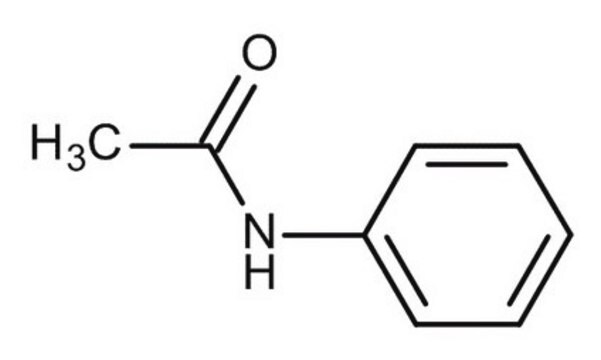
4-Nitroacetanilide
98%
Synonym(s):
4′-Nitroacetanilide, Acetic acid 4-nitroanilide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
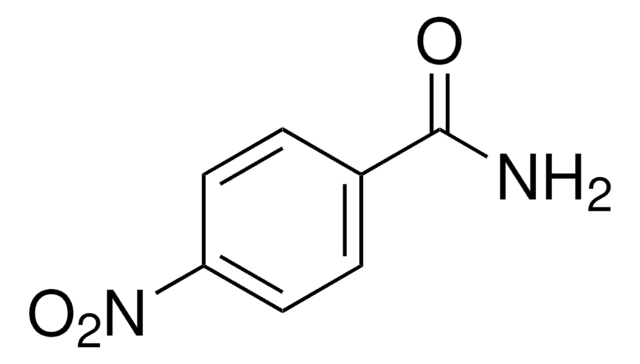
CH3CONHC6H4NO2
CAS Number:
Molecular Weight:
180.16
Beilstein:
2211962
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
213-215 °C (lit.)
functional group
amide
SMILES string
CC(=O)Nc1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H8N2O3/c1-6(11)9-7-2-4-8(5-3-7)10(12)13/h2-5H,1H3,(H,9,11)
InChI key
NQRLPDFELNCFHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Nitroacetanilide was used as a test substrate and its hydrolysis was determined by UV spectroscopic measurements. It was also used to prepare 4-aminoacetanilide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
María F Montenegro et al.
Biological chemistry, 389(4), 425-432 (2008-01-23)
Apart from its esterase activity, butyrylcholinesterase (BuChE) displays aryl acylamidase (AAA) activity able to hydrolyze o-nitroacetanilide (ONA) and its trifluoro-derivative (F-ONA). We report here that, despite amidase and esterase sites residing in the same protein, in human samples depleted of
Short-column liquid chromatographic assay for caffeine and chloramphenicol in serum.
R S Markin et al.
Journal of chromatography, 525(2), 464-470 (1990-02-23)
Hydrolysis of aromatic amides as assay for carboxylesterases-amidases.
E Heymann et al.
Methods in enzymology, 77, 405-409 (1981-01-01)
Shinji Takenaka et al.
Journal of bioscience and bioengineering, 107(1), 27-32 (2009-01-17)
Bacillus cereus strain 10-L-2 synthesizes two arylamine N-acetyltransferases (Nat-a and Nat-b) with broad substrate specificities toward aniline and its derivatives. In southern blot analysis using probes encoding the NH2-terminus of Nat-b and a conserved region of N-acetyltransferases, digested total DNA
Dirk Volkmer et al.
Inorganic chemistry, 35(13), 3792-3803 (1996-06-19)
Dinuclear nickel(II) complexes of the ligands 2,6-bis[bis((2-benzimidazolylmethyl)amino)methyl]-p-cresol (bbapOH), N,N,N',N'-tetrakis(2-benzimidazolylmethyl)-2-hydroxy-1,3-diaminopropane (tbpOH), N-methyl-N,N',N'-tris(2-benzimidazolylmethyl)-2-hydroxy-1,3-diaminopropane (m-tbpOH) and 1-[N,N-bis(2-benzimidazolylmethyl)amino]-3-[2-(3,5-dimethyl-1H-pyrazol-1-yl)ethoxy]-2-hydroxypropane (bpepOH) were prepared in order to model the active site of urease. The novel asymmetric structures of the dinuclear complexes were characterized by X-ray structure analysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service