All Photos(2)
About This Item
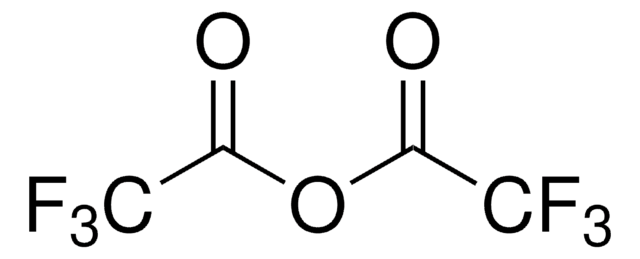
Linear Formula:
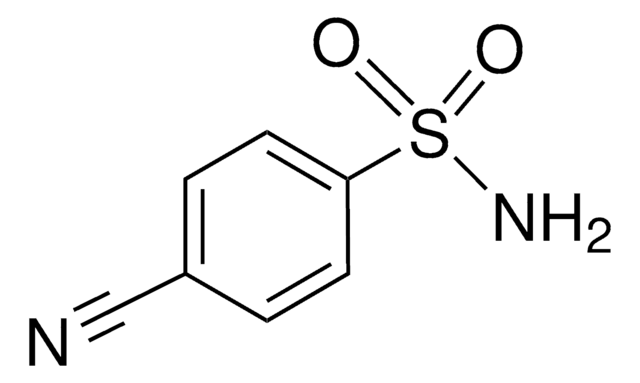
O2NC6H4SO2NH2
CAS Number:
Molecular Weight:
202.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
178-180 °C (lit.)
functional group
nitro
SMILES string
NS(=O)(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H6N2O4S/c7-13(11,12)6-3-1-5(2-4-6)8(9)10/h1-4H,(H2,7,11,12)
InChI key
QWKKYJLAUWFPDB-UHFFFAOYSA-N
Related Categories
General description
4-Nitrobenzenesulfonamide is the nitrene source during on pot procedure for copper(I)-catalyzed asymmetric alkene aziridination. It reacts with diazacrown ether, N,N′-dibenzyl-1,7,10,16-tetraoxo-4,13-diazacyclooctadecane to form molecular complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper (I)-catalyzed asymmetric alkene aziridination mediated by PhI (OAc)< sub> 2</sub>: a facile one-pot procedure.
Kwong HL, et al.
Tetrahedron Letters, 45(20), 3965-3968 (2004)
The 1: 2 and 1: 1 molecular complexes of N,N'-dibenzyl-4, 13-diaza-18-crown-6 with 4-nitrobenzenesulfonamide and dithiooxamide.
Fonari MS, et al.
Journal of Molecular Structure, 794(1), 110-114 (2006)
Jonathan T Park et al.
Chembiochem : a European journal of chemical biology, 16(5), 811-818 (2015-02-24)
Nitroreductases (NRs) and ene-reductases (ERs) both utilize flavin mononucleotide cofactors but catalyze distinct reactions. NRs reduce nitroaromatics, whereas ERs reduce unsaturated C=C double bonds, and these functionalities are known to somewhat overlap. Recent studies on the ER xenobiotic reductase A
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 120502-5G | 4061838714251 |
| 120502-25G | 4061832418506 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service