P4809
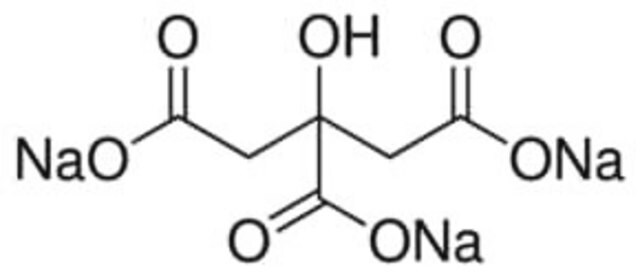
Phosphate-Citrate Buffer
tablet
Synonym(s):
PCR Buffer
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12161700
NACRES:
NA.25
Recommended Products
form
tablet
pH
4.5-5.5 (1 tablet/100mL in water)
solubility
water: soluble
storage temp.
room temp
General description
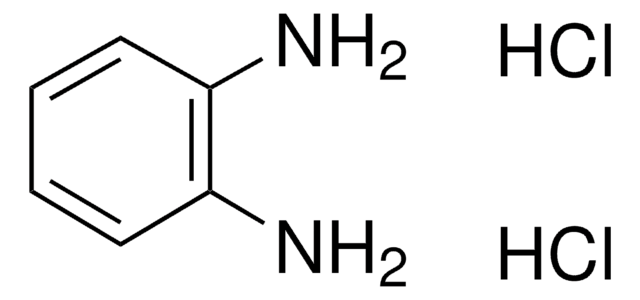
Phosphate-citrate buffer is a common buffer used along with soluble horseradish peroxidase substrates such as o-phenylenediaminedihydrochloride (OPD) or tetramethylbenzidine (TMB). It gives a pH of 5.0 with an excellent buffering capacity. The phosphate-citrate buffer tablets can be used to prepare a 0.05 M phosphate-citrate, pH 5.0, buffer solution. These tablets elicit a fast, convenient, and accurate method for the synthesis of phosphate citrate buffer solutions. They remove the time-consuming and tedious process of weighing individual components in buffer preparation. Phosphate-citrate buffer tablets have been manufactured from the highest quality components to exacting physical and chemical specifications to ensure performance and lot-to-lot consistency. Phosphate-Citrate Buffer tablets, pH 5.0, have been formulated as a substrate buffer in immunoassay procedures.
Application
- Characterization of nettle leaves (Urtica dioica) as a novel source of protease for clotting dromedary milk by non-destructive methods.: This study uses phosphate-citrate buffer in the enzymatic extraction processes to maintain stability and activity of plant-derived enzymes, demonstrating its crucial role in the development of natural coagulants for dairy products (Bouazizi et al., 2022).
- A rapid, low pH, nutrient stress, assay to determine the bactericidal activity of compounds against non-replicating Mycobacterium tuberculosis.: Utilizing phosphate-citrate buffer, this study develops a low pH nutrient stress assay for evaluating the efficacy of antibacterial agents against dormant tuberculosis bacteria, providing a critical tool for drug development (Early et al., 2019).
- Measurement of Free Iodine in Different Formulations of Povidone-Iodine Eye Drops 5.: This investigation uses phosphate-citrate buffer to measure the stability and release of iodine in ophthalmic solutions, highlighting its importance in ensuring the efficacy and safety of eye care products (Prado et al., 2019).
Reconstitution
One tablet dissolved in 100 mL deionized water yields a 0.05 M phosphate-citrate buffer, pH 5.0, at 25 °C. Store at room temperature.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rose, N., et al.
Manual of Clinical Laboratory Immunology, 106-106 (1986)
In vivo experimental model of orthotopic dental pulp regeneration under the influence of photobiomodulation therapy.
Maria Stella Moreira et al.
Journal of photochemistry and photobiology. B, Biology, 166, 180-186 (2016-12-09)
Antonino Tuttolomondo et al.
Oncotarget, 8(37), 61415-61424 (2017-10-06)
Anderson-Fabry disease (AFD) is an inborn lysosomal enzymopathy resulting from the deficient or absent activity of the lysosomal exogalactohydrolase, α-galactosidase A. This deficiency, results in the altered metabolism of glycosphingolipids which leads to their accumulation in lysosomes, thus to cellular
A S de Mello et al.
Journal of biomedicine & biotechnology, 2011, 132581-132581 (2011-06-11)
The Epstein-Barr virus (EBV) was used as an agent of B lymphocyte proliferation for subsequent diagnosis of lysosomal storage disease. Due to the constant handling of long-preserved samples in our cell bank, we decided to observe the behavior and then
Giuseppe Mangialardi et al.
Diabetologia, 62(7), 1275-1290 (2019-04-20)
Previous studies have shown that diabetes mellitus destabilises the integrity of the microvasculature in different organs by damaging the interaction between pericytes and endothelial cells. In bone marrow, pericytes exert trophic functions on endothelial cells and haematopoietic cells through paracrine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service