C9375
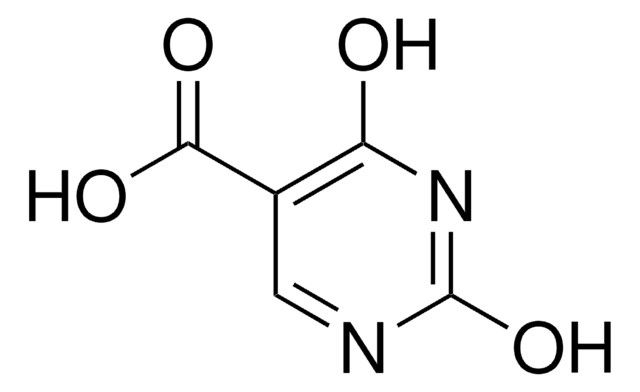
5-Carboxy-2-thiouracil
Synonym(s):
2-Thiouracil-5-carboxylic acid, 5-Carboxy-4-hydroxy-2-thiopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H4N2O3S
CAS Number:
Molecular Weight:
172.16
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98% (TLC)
form
powder
solubility
1 M NaOH: 50 mg/mL, clear, colorless to faintly yellow
SMILES string
OC(=O)c1cnc(S)nc1O
InChI
1S/C5H4N2O3S/c8-3-2(4(9)10)1-6-5(11)7-3/h1H,(H,9,10)(H2,6,7,8,11)
InChI key
XKHWTDCFQVKNHW-UHFFFAOYSA-N
Application
5-Carboxy-2-thiouracil in metal complexes with Mn(ll), Co(ll), Ni(ll), Cu(ll), Zn(ll) and Cd(ll) ions may be useful in some anti-tumor applications. 5-Carboxy-2-thiouracil may be used to produce potential anti-bacterial and anti-tumor methylhydrazonium salts.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
U P Singh et al.
Metal-based drugs, 5(1), 35-39 (2008-05-14)
Metal complexes of 5-carboxy-2-thiouracil with Mn(ll), Co(ll), Ni(ll), Cu(ll), Zn(ll) and Cd(ll) ions were synthesized, characterized, and subjected to a screening system for evaluation of antitumour activity against Sarcoma-180 (S-180) tumour cells. The complexes were characterized by elemental analysis, infrared
E Golovinsky et al.
Die Pharmazie, 37(5), 355-356 (1982-05-01)
On reacting pyrimidine metabolites containing a carboxyl or sulfhydryl group with methylhydrazine or N-benzyl-N'-methylhydrazine, various methylhydrazonium salts of these metabolites were synthetized, e.g., the salts of 5-fluororotic acid, 5-azaorotic acid, 2-thiouracil-5-carboxylic acid and 2-thio-6-azathymine. Some of these salts exhibited a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service