B8271
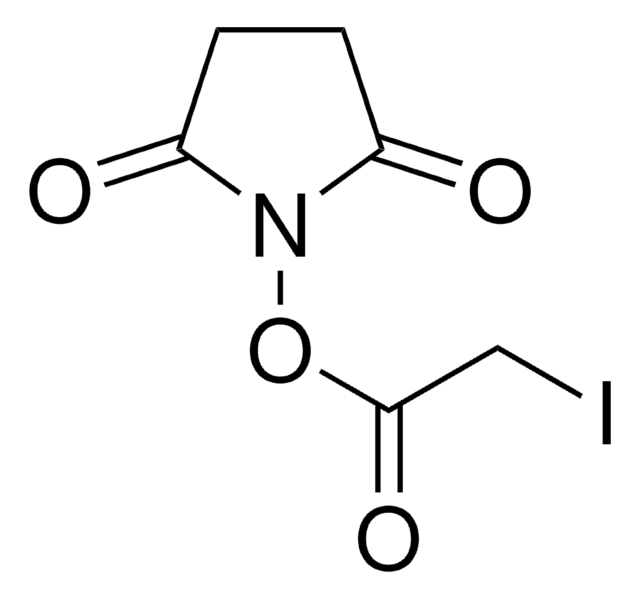
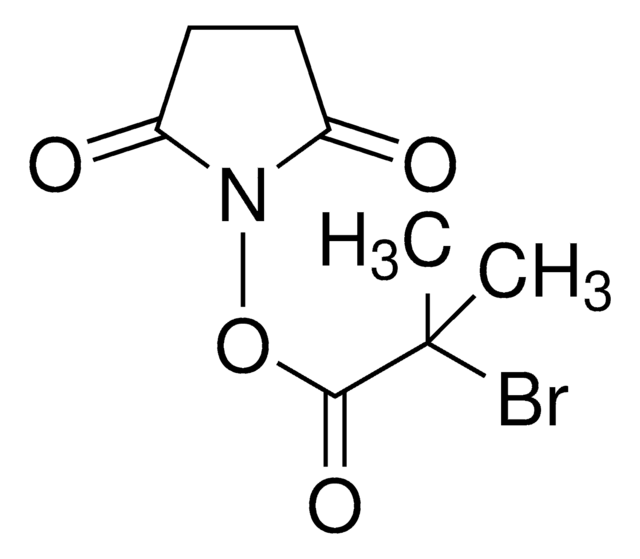
Bromoacetic acid N-hydroxysuccinimide ester
≥95%, powder
Synonym(s):
2,5-Dioxopyrrolidin-1-yl 2-bromoacetate, N-Hydroxysuccinimide bromoacetate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H6BrNO4
CAS Number:
Molecular Weight:
236.02
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NC.07
Recommended Products
Quality Level
Assay
≥95%
form
powder
reaction suitability
reagent type: cross-linking reagent
solubility
acetone: 25 mg/mL
DMF: soluble
functional group
NHS ester
shipped in
dry ice
storage temp.
−20°C
SMILES string
O=C(N1OC(CBr)=O)CCC1=O
InChI
1S/C6H6BrNO4/c7-3-6(11)12-8-4(9)1-2-5(8)10/h1-3H2
InChI key
NKUZQMZWTZAPSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
A heterobifunctional cross-linking reagent which allows bromoacetylation of primary amine groups followed by coupling to sulfhydryl-containing compounds. Typically, initial reaction couples via ester to primary amine by amide bond formation in the pH range 6.5-8.5. The second reaction results in thioether bonding in pH range 7.0-8.0.
Caution
The bromoacetyl group is light sensitive.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Kolodny et al.
Analytical biochemistry, 187(1), 136-140 (1990-05-15)
A method described here for conjugating synthetic peptides to carrier proteins provides a convenient method for determining peptide-to-carrier protein ratios. N-Bromoacetyl-containing peptides are reacted in situ with carrier proteins in which the disulfide bonds were reduced with tri-n-butylphosphine. At pH
M S Bernatowicz et al.
Analytical biochemistry, 155(1), 95-102 (1986-05-15)
Synthetic peptides derived from human fibrin were unidirectionally conjugated to three carrier proteins (bovine serum albumin, bovine alpha-lactalbumin, and keyhole limpet hemocyanin) by a method that employs N-succinimidyl bromoacetate. This heterobifunctional crosslinking reagent was prepared with a 79% yield in
John S Mort et al.
Methods in molecular medicine, 100, 237-250 (2004-07-29)
The use of synthetic peptides to generate rabbit polyclonal anticatabolic neoepitope antibodies that can be used to study the presence of defined proteolytic cleavage sites in aggrecan is described. Principles of peptide design and methods for preparation and characterization of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service