38790
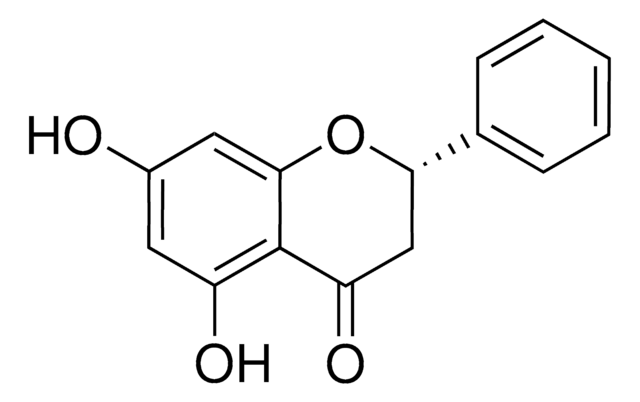
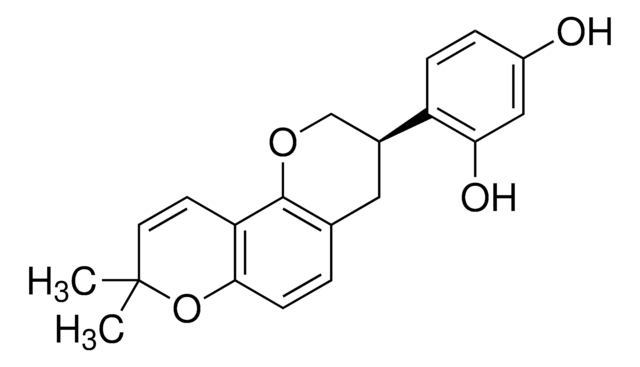
(±)-Pinostrobin
≥99.0% (TLC)
Synonym(s):
(RS)-2,3-Dihydro-5-hydroxy-7-methoxy-2-phenyl-4H-1-benzopyran-4-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H14O4
CAS Number:
Molecular Weight:
270.28
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥99.0% (TLC)
SMILES string
COc1cc(O)c2C(=O)CC(Oc2c1)c3ccccc3
InChI
1S/C16H14O4/c1-19-11-7-12(17)16-13(18)9-14(20-15(16)8-11)10-5-3-2-4-6-10/h2-8,14,17H,9H2,1H3
InChI key
ORJDDOBAOGKRJV-UHFFFAOYSA-N
Application
(±)-Pinostrobin, a chiral flavonoid (±), may be used in the development of analytical methods for separation of its individual enantiomers. Pinostrobin may be used to study its neuroprotective antioxidant effects.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alka Jaudan et al.
PloS one, 13(2), e0191523-e0191523 (2018-02-09)
Pinostrobin (PN) is a naturally occurring dietary bioflavonoid, found in various medicinal herbs/plants. Though anti-cancer potential of many such similar constituents has been demonstrated, critical biochemical targets and exact mechanism for their apoptosis-inducing actions have not been fully elucidated. The
Yan-Fang Xian et al.
Cellular and molecular neurobiology, 32(8), 1223-1230 (2012-05-09)
Beta-Amyloid peptide (Aβ), a major protein component of brain senile plaques in Alzheimer's disease (AD), has been considered as a critical cause in the pathogenesis of AD. Pinostrobin, a potent flavonoid inducer, is the major and most active ingredient of
Monika Asztemborska et al.
Electrophoresis, 24(15), 2527-2531 (2003-08-06)
Micellar electrokinetic chromatography (MEKC) was applied for enantioseparation of selected flavanones, including naringin, hesperidin, neohesperidin, naringenin, hesperetin, pinostrobin, isosakuranetin, eriodictyol, and homoeriodictyol. gamma-Cyclodextrin (gamma-CD) and sodium cholate (SCh) were used as chiral modifiers inducing enantioselectivity to the background electrolyte. From
Stereospecific analytical method development and preliminary in vivo pharmacokinetic characterization of pinostrobin in the rat.
Sayre CL, Zhang Y, Martinez SE, et al.
Biomedical Chromatography (2012)
Karen J Marsh et al.
Phytochemistry, 160, 31-39 (2019-01-27)
A group of plant specialised metabolites (PSMs) collectively known as unsubstituted B-ring flavanones (UBFs) have previously been found in the foliage of some species from the genus Eucalyptus L'Hér. (Myrtaceae), specifically from the subgenus Eucalyptus (monocalypts). Captive feeding studies using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service