07036
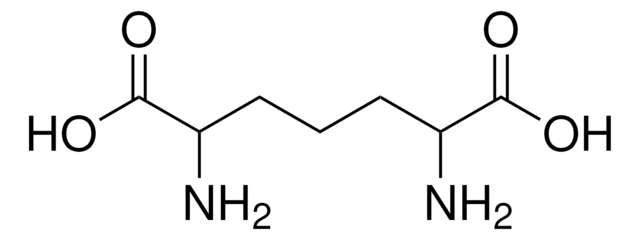
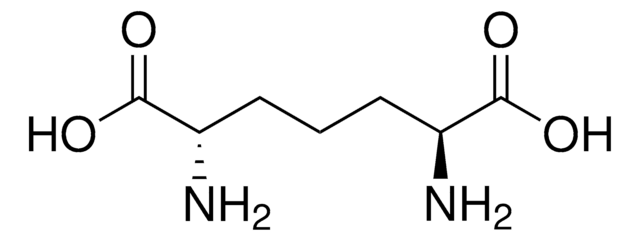
meso-2,6-Diaminopimelic acid
≥98% (TLC)
Synonym(s):
(2SR, 6RS)-2,6-Diaminoheptanedioic acid, meso-DAP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14N2O4
CAS Number:
Molecular Weight:
190.20
Beilstein:
1726899
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98% (TLC)
form
powder
color
white to faint beige
application(s)
cell analysis
SMILES string
N[C@H](CCC[C@H](N)C(O)=O)C(O)=O
InChI
1S/C7H14N2O4/c8-4(6(10)11)2-1-3-5(9)7(12)13/h4-5H,1-3,8-9H2,(H,10,11)(H,12,13)/t4-,5+
InChI key
GMKMEZVLHJARHF-SYDPRGILSA-N
Related Categories
Application
Meso-2,6-diaminopimelic acid may be used to study peptidoglycan structure and function within the cell walls of bacteria.
Biochem/physiol Actions
Penultimate biosynthetic precursor of the essential amino acid L-lysine. Component of peptidoglycan in the cell wall of many bacteria.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Satyanarayana et al.
Amino acids, 21(3), 221-235 (2002-01-05)
Peptidoglycans isolated from two Fusobacterium species of anaerobic bacteria were analyzed for constituent amino acids. Hydrolysis conditions were varied to optimize the yield of diamino acids from peptidoglycan. The o-phthalaldehyde derivatives of the diamino acid stereoisomers were separated by high-performance
Luigi Franchi et al.
Cellular microbiology, 10(1), 1-8 (2007-10-20)
The innate immune system comprises several classes of pattern-recognition receptors, including Toll-like receptors (TLRs) and nucleotide binding and oligomerization domain-like receptors (NLRs). TLRs recognize microbes on the cell surface and in endosomes, whereas NLRs sense microbial molecules in the cytosol.
Chiral high-performance liquid chromatographic separation of the three stereoisomers of 2,6-diaminopimelic acid without derivatization.
Nagasawa, T., et al.
Journal of Chromatography A, 653, 336-340 (1993)
N V Potekhina et al.
European journal of biochemistry, 199(2), 313-316 (1991-07-15)
The teichoic acid from the cell wall of Actinomadura cremea INA 292 has an unusual structure, being a poly(galactosylglycerol phosphate) chain with glycerol phosphate groups. Monomeric units of 1-O, beta-D-galactopyranosylglycerol monophosphate are joined in the polymer by phosphodiester links involving
Yukari Fujimoto et al.
Methods in enzymology, 478, 323-342 (2010-09-08)
In this chapter, we describe synthetic studies on partial structures of lipopolysaccharide (LPS) and peptidoglycan (PGN), which work as tags for nonself recognition in innate immune system. Our previous studies demonstrated that lipid A is the endotoxic principle of LPS.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service