Y0000612
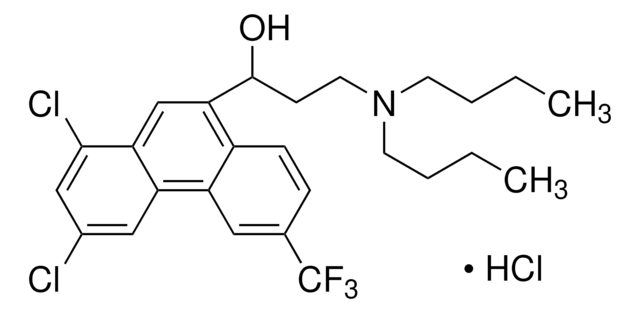
Dopexamine dihydrochloride
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H32N2O2 · 2HCl
CAS Number:
Molecular Weight:
429.42
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
dopexamine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C22H32N2O2.2ClH/c25-21-11-10-20(18-22(21)26)13-17-24-15-7-2-1-6-14-23-16-12-19-8-4-3-5-9-19;;/h3-5,8-11,18,23-26H,1-2,6-7,12-17H2;2*1H
InChI key
VPDULUNRSQWWJB-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Dopexamine dihydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Georgios I Tagarakis et al.
Recent patents on cardiovascular drug discovery, 5(1), 66-68 (2009-11-26)
A great variety of inotropic agents with different effects on peripheral vascular resistance have been employed in the endeavor to treat heart failure after cardiac surgery. Epinephrine, norepinephrine, dopamine and dobutamine belong to the first-line, widely used drugs recruited to
Han-Ming Chen et al.
Hepato-gastroenterology, 53(67), 39-44 (2006-03-02)
This work examines the effects of lipopolysaccharide (LPS) on splanchnic blood flow and tests the potential effect of dopexamine in preventing LPS-induced decrease in splanchnic blood flow, also analyzing its influence on regional leukotriene production. Male Sprague-Dawley rats were grouped
S Gopal et al.
Anaesthesia, 64(6), 589-594 (2009-05-21)
The objective of the study was to determine whether dopexamine alters in-hospital mortality. The following databases were searched, Embase (1974-July 2007), Medline (1950-July 2007), CINAHL, PubMed and Cochrane Clinical Register of Controlled Trials (CENTRAL). Two reviewers independently checked the quality
Ayten Bilir et al.
Saudi medical journal, 27(8), 1194-1198 (2006-08-03)
To compare the inotropic and chronotropic effects of ropivacaine and bupivacaine in an isolated, spontaneously beating rat heart, and to determine the reversal effects of dopexamine on these effects. The study was conducted at the Department of Physiology, Medical Faculty
D Rapp-Kesek et al.
Acta anaesthesiologica Scandinavica, 51(5), 570-576 (2007-04-14)
After cardiac surgery, patients are at risk of organ dysfunction because of decreased perfusion. Different measures have been used to increase the splanchnic blood flow. We compared the effects of enteral nutrition and dopexamine on the cardiac output, splanchnic blood
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service