S0947000
Somatropin
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
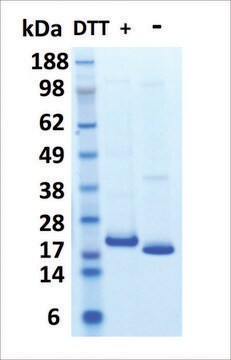
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
somatropin
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Somatropin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Elowe-Gruau et al.
Revue medicale suisse, 10(418), 424-424 (2014-03-20)
Children born premature and/or small for gestational age (SGA) are at risk of growth and metabolic abnormalities. Catch-up growth occurs usually before the age of 2. In the absence of sufficient catch up growth, growth hormone (GH) treatment should be
Małgorzata Partyka et al.
Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego, 36(211), 63-67 (2014-03-22)
Growth hormone (GH) is a polypeptide hormone produced by the cells of pituitary. Production of growth hormone is carried out in a pulsating manner, and the frequency and intensity of the pulses is dependent on age and gender. Growth hormone
A Thankamony et al.
The Journal of clinical endocrinology and metabolism, 99(2), 639-647 (2014-01-16)
Data on the metabolic effects of GH derived from studies using GH suppression by pharmacological agents may not reflect selective actions. The purpose of this study was to evaluate the effects of GH antagonism on glucose and lipid metabolism using
Mark Sherlock et al.
The Journal of clinical endocrinology and metabolism, 99(2), 478-485 (2013-11-19)
Acromegaly is associated with reduced life expectancy, which has been reported to be normalized if treatment is successful in controlling GH/IGF-I levels. Most previous studies have invariably used the last available GH/IGF-I, which may be biased as it only assesses
J R Delanghe et al.
Acta clinica Belgica, 69(1), 25-29 (2014-03-19)
The recent Armstrong case, where more than 250 negative doping tests are confronted with the athlete's confession of erythropoietin use, blood doping, steroid, and growth hormone abuse, illustrates the limitations of current laboratory tests in detecting doping in sport. Despite
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service