924377
Palladium(II) Acetate ChemBeads
Synonym(s):
Acetic acid palladium salt, Bis(acetato)palladium, Diacetatopalladium, Diacetoxypalladium
About This Item
Recommended Products
form
solid
Quality Level
composition
~ 4 wt.% loading of catalyst
reaction suitability
core: palladium
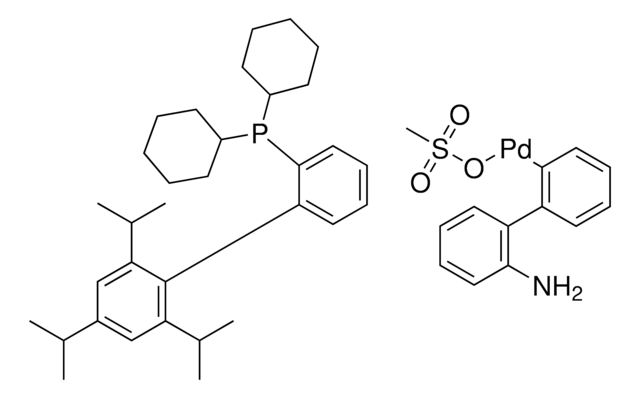
reaction type: Buchwald-Hartwig Cross Coupling Reaction
core: palladium
reaction type: Heck Reaction
core: palladium
reaction type: Hiyama Coupling
core: palladium
reaction type: Negishi Coupling
core: palladium
reaction type: Sonogashira Coupling
core: palladium
reaction type: Stille Coupling
core: palladium
reaction type: Suzuki-Miyaura Coupling
reaction type: Cross Couplings
reagent type: catalyst
InChI
1S/2C2H4O2.Pd/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
YJVFFLUZDVXJQI-UHFFFAOYSA-L
General description
Application
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
Learn more about ChemBeads products
For larger scale uses, product also available in powdered form (205869) & (520764)
Other Notes
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service