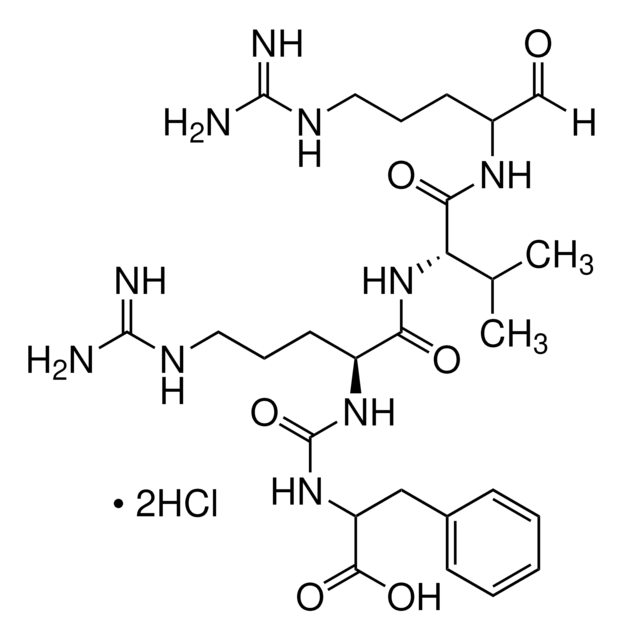
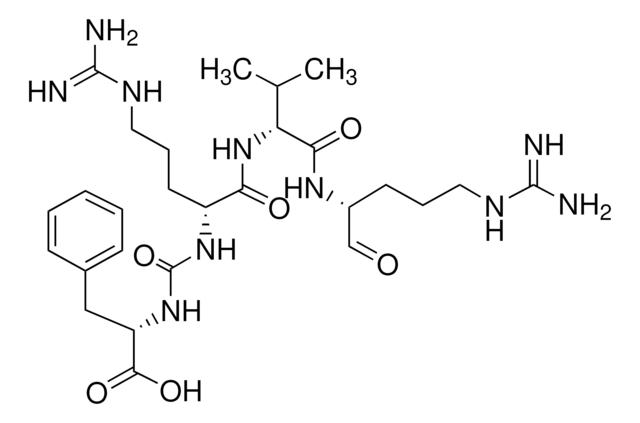
Recommended Products
General description
Antipain is known for regulating the number of chromosomal aberrations caused by the S-phase-dependent alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine. It can also diminish the nuclear binding of the estrogen-receptor complex in MCF-7 breast tumor cells.
Application
Antipain has been used in microfluidic chip assay.
Biochem/physiol Actions
Inhibitor Type: Protease Inhibitors
Quality
pH 1% aqueous solution: 5.2
Physical form
Lyophilized no preservatives.
Properties: Soluble in H2O, methanol and DMSO (Stock solution: 10 mM). Stable at -20° C.
Mechanism of action: Formation of a hemicetal adduct between the aldehyde group of the inhibitor and the active serine of the proteinase.
Inhibition spectrum: Inhibits papain, trypsin and plasmin to a lesser extent. More specific for papain and trypsin than leupeptin. The inhibitory potency of antipain is 100-fold higher than that of elastatinal.
Pale yellow powder; material is isolated from cultured media of microorganisms. As with many of these compounds, they are too inherently unstable to allow for high standards of purity. The homogeneity of these compounds cannot be guaranteed except for their specific inhibitory activities.
Properties: Soluble in H2O, methanol and DMSO (Stock solution: 10 mM). Stable at -20° C.
Mechanism of action: Formation of a hemicetal adduct between the aldehyde group of the inhibitor and the active serine of the proteinase.
Inhibition spectrum: Inhibits papain, trypsin and plasmin to a lesser extent. More specific for papain and trypsin than leupeptin. The inhibitory potency of antipain is 100-fold higher than that of elastatinal.
Pale yellow powder; material is isolated from cultured media of microorganisms. As with many of these compounds, they are too inherently unstable to allow for high standards of purity. The homogeneity of these compounds cannot be guaranteed except for their specific inhibitory activities.
Storage and Stability
Maintain dry at -20ºC for up to 18 months. Store reconstituted product in aliquots at -20ºC for up to 9 months. Do not thaw and refreeze.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure of antipain, a new Sakaguchi-positive product of streptomyces.
S Umezawa et al.
The Journal of antibiotics, 25(4), 267-270 (1972-04-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service