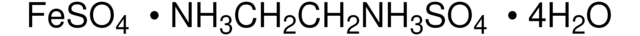1.09092
Cerium(IV) sulfate solution
c(Ce(SO₄)₂ * 4 H₂O) = 0.1 mol/l (0.1 N), reag. Ph. Eur., ready-to-use volumetric solution for titration, Titriplex®
Synonym(s):
Cer sulfate, Cer(IV) sulfate
About This Item
Recommended Products
Product Name
Cerium(IV) sulfate solution, c(Ce(SO4)2 * 4 H2O) = 0.1 mol/l (0.1 N), Titripur®, reag. Ph. Eur.
Agency
reag. Ph. Eur.
Quality Level
product line
Titripur®
form
liquid
quality
Analyzed in our ISO 17025 accredited QC lab
reaction suitability
reaction type: Redox Reactions
concentration
0.1 M
technique(s)
titration: suitable
pH
0.4 (20 °C in H2O)
density
1.06 g/cm3 at 20 °C
storage temp.
15-25°C
Application
- Humic acid removal using cellulose acetate membranes grafted with poly (methyl methacrylate) and aminated using tetraethylenepentamine.: This study highlights the application of cellulose acetate membranes modified with polymers for the efficient removal of humic acids from water, employing Cerium(IV) sulfate as a catalyst to initiate the polymerization process, which significantly enhances the membrane′s performance in water treatment (Gebru KA et al., 2018).
- Characterization and ecological risk assessment of nanoparticulate CeO2 as a diesel fuel catalyst.: This research provides a detailed characterization of Cerium(IV) sulfate used as a precursor for nanoparticulate CeO2, assessing its ecological risks when applied as a catalyst in diesel fuels, offering insights into its environmental impact (Batley GE et al., 2013).
- Chemiluminescence from an oxidation reaction of rhodamine B with cerium(IV) in a reversed micellar medium of cetyltrimethylammonium chloride in 1-hexanol-cyclohexane/water.: Explores the enhanced chemiluminescence reactions of Cerium(IV) sulfate, demonstrating its role in improving the sensitivity and specificity of analytical methods for detecting organic compounds (Hasanin TH et al., 2011).
- Chemiluminescence reactions with cationic, neutral, and anionic ruthenium(II) complexes containing 2,2′-bipyridine and bathophenanthroline disulfonate ligands.: Investigates the chemiluminescence properties of various ruthenium(II) complexes, where Cerium(IV) sulfate serves as an effective oxidizing agent, highlighting its potential in analytical applications for detecting and measuring trace amounts of biological and chemical substances (Francis PS et al., 2010).
- Chemiluminescence method for the determination of piroxicam by the enhancement of the tris-(4,7-diphenyl-1,10-phenanthrolinedisulphonic acid) ruthenium(II) (RuBPS)-cerium(IV) system and its application.: Presents a novel chemiluminescence technique for determining piroxicam, utilizing the catalytic properties of Cerium(IV) sulfate to enhance the luminescence signal, thereby offering a sensitive analytical tool for pharmaceutical analysis (Yu F et al., 2009).
Features and Benefits
This volumetric solution is analyzed by our calibration laboratory D-K-15185-01-00 which is accredited according to DIN EN ISO/IEC 17025 for analysis of amount-of-substance concentrations in volumetric solutions by DAkkS (Deutsche Akkreditierungsstelle - German National Accreditation Body). The accreditation certificate can be found at www.sigmaaldrich.com/ISO17025.
Packaging
Linkage
Analysis Note
Amount-of-substance concentration 0.0995 - 0.1005 mol/L
Measurement uncertainty ± 0.0003 mol/L
Traceability NIST SRM
The concentration is determined by volumetric titration and refers to 20°C.
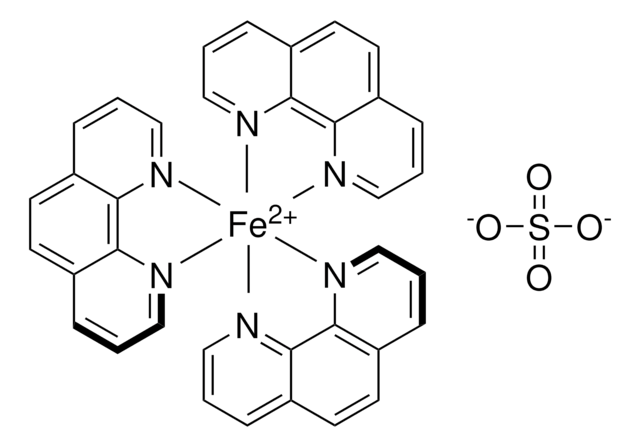
The amount-of-substance concentration of this volumetric solution is traceable to a primary standard reference material (SRM) from the National Institute of Standards and Technology, Gaithersburg, USA (NIST SRM 8040 di-sodium oxalate) by means of volumetric standard Iron(II)ethylendiammonium sulfate (article number 1.02402), certified reference material according to ISO 17034, analyzed by our accredited calibration laboratory of Merck KGaA, Darmstadt, Germany according to DIN EN ISO/IEC 17025. The uncertainty is expressed as expanded measurement uncertainty with a coverage factor k=2 covering a confidence level of 95%.
Note: The titer is a correction factor to correct for variations of the volumetric solution, the titration equipment, the temperature and other laboratory conditions. For correct titration results it is recommended to determine a titer with the laboratory specific equipment and under laboratory specific conditions directly after opening a new bottle and at regular time intervals.
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service