07-482
Anti-Calmodulin Binding Protein Epitope Tag Antibody
Upstate®, from rabbit
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
rabbit
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
polyclonal
purified by
affinity chromatography
species reactivity
E. coli
manufacturer/tradename
Upstate®
technique(s)
western blot: suitable
isotype
IgG
shipped in
wet ice
target post-translational modification
unmodified
General description
Calmodulin (CaM), a small 17 kDa ubiquitous Ca2+-binding protein acts as an intracellular Ca2+ receptor and transduces Ca2+ transients, usually in response to growth factors. It mediates the activity of a number of Ca2+ regulating enzymes, including protein kinases, phosphatases, nitric oxide synthases, and phosphodiesterase.
Specificity
Recognizes recombinant proteins containing the calmodulin binding protein epitope tag.
Immunogen
KLH-conjugated, synthetic peptide (MKRRWKKNFIAVSAANRFKKISSSGAL) corresponding to the Calmodulin Binding Protein (CBP) epitope tag found in several E. coli expression vectors. The immunizing sequence is identical in rabbit, mouse, and rat.
Application
Anti-Calmodulin Binding Protein Epitope Tag Antibody detects level of Calmodulin Binding Protein Epitope Tag & has been published & validated for use in WB.
Quality
Routinely evaluated by Western Blot in lysates from transformed E. coli.
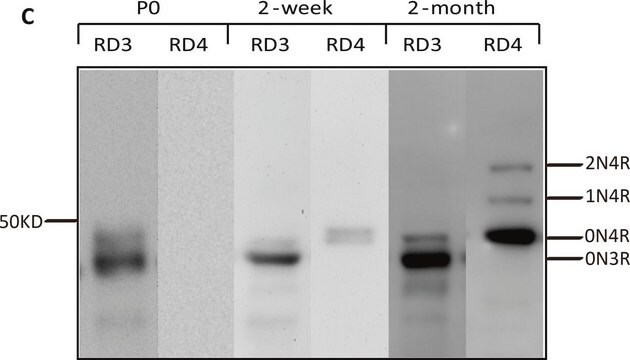
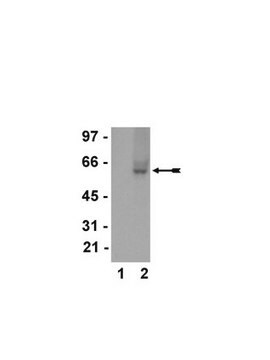
Western Blot Analysis:
A 1:2000-1:10000 dilution of this lot detected a recombinant protein containing the calmodulin binding protein epitope tag in lysates from transformed E. coli.
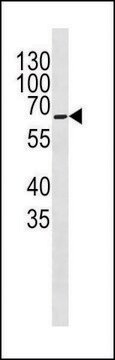
Western Blot Analysis:
A 1:2000-1:10000 dilution of this lot detected a recombinant protein containing the calmodulin binding protein epitope tag in lysates from transformed E. coli.
Target description
The molecular weight is related to the tagged protein.
Physical form
Purified rabbit IgG in buffer containing 0.1M Tris-Glycine, 0.15M NaCl, 0.05% Sodium Azide, pH7.4.
Analysis Note
Control
Lysates from transformed E. coli.
Lysates from transformed E. coli.
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas Hubert et al.
Traffic (Copenhagen, Denmark), 10(9), 1257-1271 (2009-06-26)
Nucleoporin Nup62 localizes at the central channel of the nuclear pore complex and is essential for nucleocytoplasmic transport. Through its FG-repeat domain, Nup62 regulates nuclear pore permeability and binds nuclear transport receptors. Here, we report that Nup62 interacts directly with
Stephen M T Hoke et al.
Current genetics, 56(5), 447-465 (2010-07-17)
Tra1 is a component of the Saccharomyces cerevisiae SAGA and NuA4 complexes and a member of the PIKK family, which contain a C-terminal phosphatidylinositol 3-kinase-like (PI3K) domain followed by a 35-residue FATC domain. Single residue changes of L3733A and F3744A
Weijun Chen et al.
RNA (New York, N.Y.), 20(3), 308-320 (2014-01-21)
Excision of introns from pre-mRNAs is mediated by the spliceosome, a multi-megadalton complex consisting of U1, U2, U4/U6, and U5 snRNPs plus scores of associated proteins. Spliceosome assembly and disassembly are highly dynamic processes involving multiple stable intermediates. In this
Role of km23-1 in RhoA/actin-based cell migration.
Jin, Q; Pulipati, NR; Zhou, W; Staub, CM; Liotta, LA; Mulder, KM
Biochemical and biophysical research communications null
Physiological importance and identification of novel targets for the N-terminal acetyltransferase NatB.
Caesar, R; Warringer, J; Blomberg, A
Eukaryotic Cell null
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service