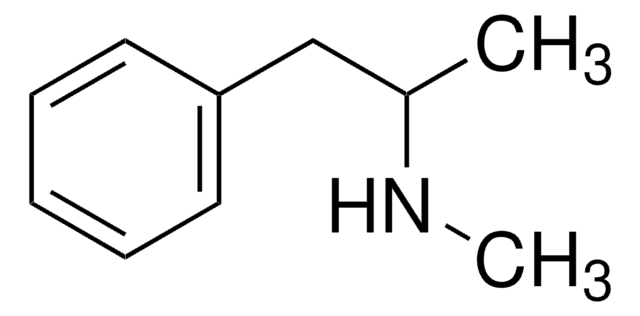
M-012
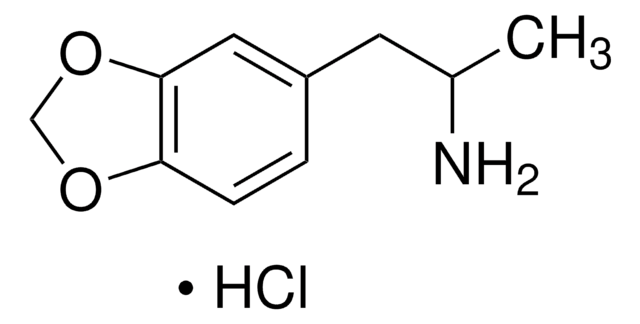
(±)-MDA solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
(±)-3,4-Methylenedioxyamphetamine
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIA (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
NC(C)CC1=CC=C(OCO2)C2=C1
InChI
1S/C10H13NO2/c1-7(11)4-8-2-3-9-10(5-8)13-6-12-9/h2-3,5,7H,4,6,11H2,1H3
InChI key
NGBBVGZWCFBOGO-UHFFFAOYSA-N
General description
Application
- MDA Research Chemical: (±)-MDA solution is extensively used in scientific research to study its pharmacological properties, including its effects on neurotransmitter systems which are critical in understanding substance-related disorders.
- MDA Enantiomers: The solution includes both enantiomers of MDA, allowing for detailed studies on the differential biological activities of each enantiomer in neuropharmacological research.
- Synthetic MDA Solution: This synthetic solution is crucial for controlled studies in biochemistry and pharmacology, providing a consistent baseline for experimental reproducibility and validation.
- MDA Biochemistry Application: Utilized in biochemical assays, (±)-MDA solution helps in the exploration of its metabolic pathways and interactions with biological macromolecules, enhancing the understanding of its pharmacodynamics.
- MDA Pharmacological Studies: As a key tool in pharmacological research, (±)-MDA solution is used to investigate its potential therapeutic effects and risks, contributing to the development of safety guidelines and therapeutic protocols.
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service