T89702
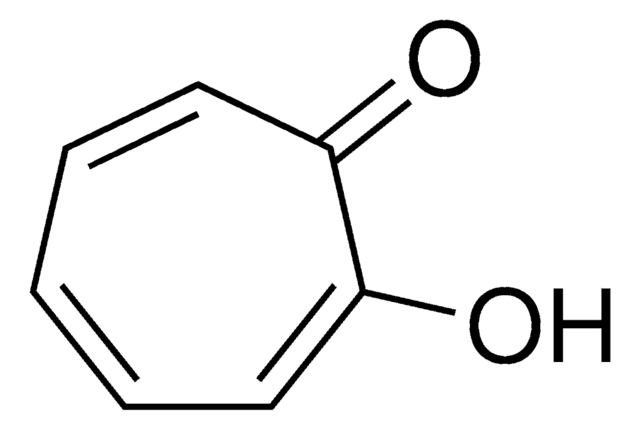
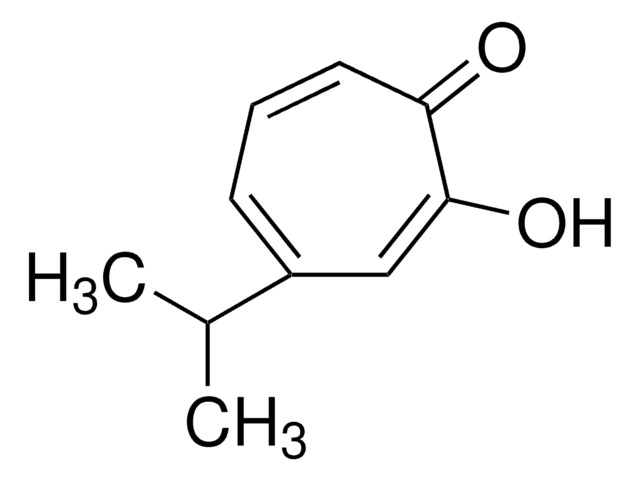
Tropolone
98%
Synonym(s):
2-Hydroxy-2,4,6-cycloheptatrien-1-one
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H6O2
CAS Number:
Molecular Weight:
122.12
Beilstein:
1904978
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
80-84 °C/0.1 mmHg (lit.)
mp
50-52 °C (lit.)
storage temp.
2-8°C
SMILES string
OC1=CC=CC=CC1=O
InChI
1S/C7H6O2/c8-6-4-2-1-3-5-7(6)9/h1-5H,(H,8,9)
InChI key
MDYOLVRUBBJPFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Tropolone is a sensitive reagent for reducing sugars. It is also an organic chelator that can form complexes with trivalent lathanide ions (Eu3+, Gd3+, and Tb3+).
It can also be used as:
It can also be used as:
- A precursor to synthesize azulene derivatives such as methyl 2-methylazulene-1-carboxylate.
- A reagent to prepare fused heterocycles and complexes of Ga(III) and In(III).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Trends Heterocycl. Chem., 1, 137-137 (1990)
Spectroscopy and non-radiative processes in Gd3+, Eu3+ and Tb3+ tropolonates.
Santos BS, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 54(13), 2237-2245 (1998)
Inorgorganica Chimica Acta, 211, 141-141 (1993)
Jinbing Liu et al.
European journal of medicinal chemistry, 44(4), 1773-1778 (2008-06-06)
A series of alkylidenethiosemicarbazide compounds were synthesized and their inhibitory effects on the diphenolase activity of mushroom tyrosinase were evaluated. The results showed that most of the synthesized compounds exhibited significant inhibitory activities. Especially, compound 1f was found to be
Anahit Penesyan et al.
Marine drugs, 9(8), 1391-1402 (2011-09-06)
Surface-associated marine bacteria often produce secondary metabolites with antagonistic activities. In this study, tropodithietic acid (TDA) was identified to be responsible for the antibacterial activity of the marine epiphytic bacterium Pseudovibrio sp. D323 and related strains. Phenol was also produced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service