T53503
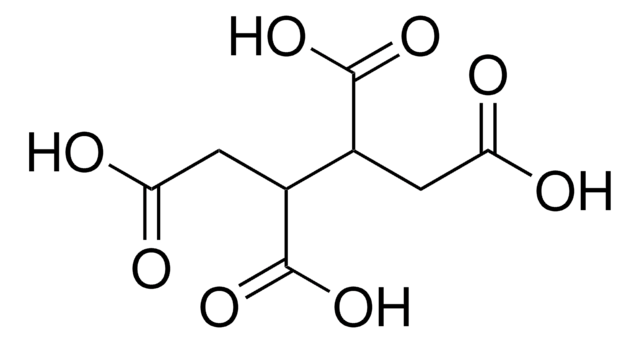
Tricarballylic acid
99%
Synonym(s):
1,2,3-Propanetricarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO2CCH2)2CHCO2H
CAS Number:
Molecular Weight:
176.12
Beilstein:
1783567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
156-161 °C (lit.)
SMILES string
OC(=O)CC(CC(O)=O)C(O)=O
InChI
1S/C6H8O6/c7-4(8)1-3(6(11)12)2-5(9)10/h3H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
InChI key
KQTIIICEAUMSDG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tricarballylic acid also known as 1,2,3-Propanetricarboxylic acid is a small biomimetic ligand that is often used as building blocks in the synthesis of metal-organic frameworks. Additionally, it is used to produce high-value plasticizers.
Application
- Effect of organic acids on the solid-state polymorphic phase transformation of piracetam.: Examines the impact of tricarballylic acid and other organic acids on the polymorphic phase transformation of the drug piracetam, relevant for pharmaceutical formulations and drug stability studies (Fan F et al., 2023).
- Nanotubule inclusion in the channels formed by a six-fold interpenetrated, triperiodic framework.: Describes the synthesis of novel materials incorporating tricarballylic acid, which could have implications for polymer production and advanced material applications (Kusumoto S et al., 2023).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Robert A Yokel et al.
Nanotoxicology, 13(4), 455-475 (2019-02-08)
Ligands that accelerate nanoceria dissolution may greatly affect its fate and effects. This project assessed the carboxylic acid contribution to nanoceria dissolution in aqueous, acidic environments. Nanoceria has commercial and potential therapeutic and energy storage applications. It biotransforms in vivo.
Jennifer A Faust et al.
The journal of physical chemistry. A, 123(10), 2114-2124 (2019-03-02)
Oxidative aging alters the composition of organic aerosols over time, in turn affecting the ability of aerosols to seed cloud formation and scatter solar radiation. Here we explore the heterogeneous photooxidation of model organic particles with and without a soluble
M Hartl et al.
The Journal of organic chemistry, 66(11), 3678-3681 (2001-05-26)
The circular dichroism (CD) exciton chirality method was employed for the stereochemical assignment of the tricarballylic acid (TCA) side chains of fumonisin B(1) 1a (FB(1)). Using 2-naphthoate for chromophoric derivatization of the reduced TCA moieties, the absolute configuration was shown
Luciana Naso et al.
Journal of inorganic biochemistry, 103(2), 219-226 (2008-11-26)
The coordination behavior of copper(II) with tricarballylic acid (H(3)TCA) and imidazole (Imz) is described. Speciation in aqueous solution has been determined at 25 degrees C and 0.15M NaCl ionic strength by potentiometric measurements and EPR characterization of the species. Two
J Krämer et al.
Journal of bacteriology, 189(11), 4290-4298 (2007-04-10)
The histidine protein kinase DcuS of Escherichia coli senses C(4)-dicarboxylates and citrate by a periplasmic domain. The closely related sensor kinase CitA binds citrate, but no C(4)-dicarboxylates, by a homologous periplasmic domain. CitA is known to bind the three carboxylate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service